Aenert. Research Laboratory news
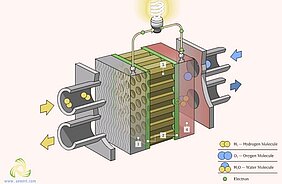
Solid oxide fuel cells (SOFC) are an emerging technology for converting chemical energy to electricity. Owing to intense research efforts dedicated to increasing the stability and performance of SOFCs, the SOFC technology is experiencing a rapidly growing interest throughout the world. Electrolysis is currently the most promising method of hydrogen production from water due to high efficiency of conversion and relatively low required energy input when compared to thermochemical and photocatalytic methods. Solid oxide electrolyser cells run in regenerative mode to achieve the electrolysis of water (and/or carbon dioxide) by using a solid oxide, or ceramic, electrolyte to produce hydrogen gas (and/or carbon monoxide) and oxygen. The general function of the electrolyzer cell is to split water in the form of steam into pure H2 and O2. Steam is fed into the porous cathode. Under the influence of steam, it moves to the cathode-electrolyte interface and is reduced to form pure H2 and oxygen ions. The hydrogen gas then diffuses back up through the cathode and is collected at its surface as hydrogen fuel, while the oxygen ions are conducted through the dense electrolyte.
Now (2023), OxEon Energy with support from NETL has drawn on NASA extraplanetary research to create a stable, robust and low-cost system capable of producing hydrogen at high pressures which means that clean energy devices are soon ready for commercialisation. OxEon’s aim was to build a solid oxide electrolysis cell (SOEC) which could produce hydrogen at elevated pressure of 2 to 3 bar. SOECs function in a similar manner to solid oxide fuel cells (SOFC) but in reverse, producing hydrogen by splitting it from water using an electric current.
This project was dedicated to addressing common challenges faced by the SOEC industry. Through modifying process and cell components, they managed to achieve improved cell performance and stability, oxidation recovery of the fuel electrode, performance stability through thermal cycles, and evaluation of the effect of contaminants.
The research consisted of testing a stack for 1,800 hours while cycling between SOEC and SOFC modes of operation. The degradation rate was 0.6% per 1,000 hours in SOEC mode and 0.3 % per 1,000 hours in SOFC mode. The SOEC electrode was also tested at Pacific Northwest National Laboratory by exposing it to steam overnight to fully oxidise the nickel component. The team was able to completely restore cell performance by simply applying voltage without providing external hydrogen gas, demonstrating oxidization recovery. It was found to be of paramount importance to keep the nickel in the hydrogen electrode in a metallic state so that it could recover after oxidation by self-generated hydrogen. Formerly, an interruption or upset in the conditions to the recycle loop could cause permanent, irreparable oxidation of the fuel electrode.
Another important achievement of the research was that the strontium-containing layer in the SOEC stack could be eliminated while retaining the same initial electrode performance as strontium precipitation, migration and reaction with other cell components are known degradation mechanisms for SOFC/SOEC operation. Testing over multiple days also showed less degradation than cells containing strontium.
Moreover, an SOEC stack tested combined the improved fuel electrode and strontium-free layer on the oxygen electrode. The stack showed complete recovery of performance after oxidation of the nickel-based fuel electrode without requiring the presence of hydrogen in the steam inlet. After five oxidation recovery cycles, the stack was deep-thermal cycled five times from the operating temperature to room temperature. The stack showed no loss in performance after each thermal cycle and retained low degradation characteristics.
Scientists are constantly working to find new methods for hydrogen production. In 2022, high-temperature (600°C) electrochemical synthesis of ammonia from N2 and H2O at atmospheric pressure in solid oxide electrolysis cell (SOEC)-type reactors was analysed. The catalytic material selection for the working electrode was found to be one of the most important challenges in electrochemical processes. In this study, a composite cathode composed of a perovskite oxide and an iron oxynitride phase was investigated. Both phases were characterised thoroughly using XRD, XPS, Mössbauer spectroscopy, TPD/TPRxn, and 4-probe electrical conductivity techniques. The electrocatalytic activity experiments were performed on the perovskite oxide phase and the composite cathode to study the effect of using a composite electrode on the activity of the cell.
Image: XRD patterns of LSF72, LSF82 and LSF92
Source: Seval Gunduz, Dhruba J. Deka, Matt Ferree, Jaesung Kim, Jean-Marc M. Millet, Anne C. Co and Umit S. Ozkan/ Composite Cathodes with Oxide and Nitride Phases for High-Temperature Electrocatalytic Ammonia Production from Nitrogen and Water/ ECS Advances, Volume 1, Number 1, 28 April 2022/ DOI 10.1149/2754-2734/ac6618/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2022, scientists analysed the setup and testing protocol for metal-supported solid oxide electrolysis cell (MS-SOEC) button cell performance evaluation. The objectives of this procedure were to define a standard testing protocol, describe materials selection, and identify common pitfalls for testing MS-SOEC button cells. The testing included preparation and operation details specific to MS-SOECs, a discussion of seal and test rig materials, alternative sealing and start-up protocols for a metal test rig with glass seal or alumina test rig with ceramic adhesive seal, and a discussion of the consequences of off-normal operation.
At the focus of the research was reproducible operation of MS-SOEC button cells at a variety of operating conditions. A pre-fabricated metal-supported solid oxide electrolysis cell was linked to a platinum or nickel mesh with conductive wire leads on the steam electrode side. Then the cell was sealed on a test rig (typically with glass paste or ceramic adhesive). Hydrogen was humidified to a specific steam content and delivered to the test rig via a heated tube. The MS-SOEC was then operated.
Image: Schematic and photograph of a MS-SOEC test setup with a heated bubbler as the humidification system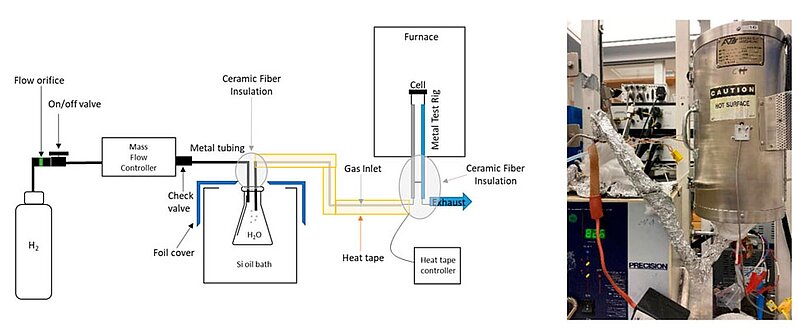
Sources: Fengyu Shen, Martha M. Welander, Michael C. Tucker/ Metal-Supported Solid Oxide Electrolysis Cell Test Standard Operating Procedure/ Front. Energy Res., Sec. Process and Energy Systems Engineering, Volume 10 – 2022, 25 April 2022/ https://doi.org/10.3389/fenrg.2022.817981/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
There are several advantages derived from hydrogen production through SOEC: The cell has improved performance and stability which will accommodate what will be required to meet the U.S. Department of Energy cost and performance targets, including achieving 40,000 operational hours. Also, these stepwise improvements to baseline performance demonstrate a pathway to low-cost hydrogen production.
In view of the energy crisis, cheap means of hydrogen production are of great interest and in high demand. International research is making its contribution to meeting the needs of a growing hydrogen economy.
By the Editorial Board