Aenert. Research Laboratory news
Copper oxide is a valuable catalyst material able to convert different feedstock such as methanol into fuels. In order to achieve an optimal result, understanding the mechanisms of the catalytic reaction started by an oxide catalyst is of great importance. This enables scientists to steer the reaction by controlling the amount of oxygen and the number of electrons involved and thus achieve a smooth and efficient reaction.
Now (2023), scientists at Brookhaven Laboratory have launched a project which is aimed at getting greater insight into how peroxides present on the surface of copper oxide are able to facilitate the oxidation of hydrogen and at the same time inhibit the oxidation of carbon monoxide. The knowledge gained should help them steer oxidation reactions better. Observing the mechanisms of these quick changes was made possible by employing different spectroscopy methods.
Peroxides contain two oxygen atoms linked by shared electrons and have relatively weak electron bonds. This makes them highly reactive. In this study, the scientists focused on altering the redox steps of catalytic oxidation reactions on an oxidized copper surface (CuO) through identifying the makeup of peroxide species formed with different gases: O2 (oxygen), H2 (hydrogen), and CO (carbon monoxide).
In a redox reaction, the oxidizing agent gains an electron and the reducing agent loses an electron. When the different peroxide species and how the altered redox steps would influence the reaction as a whole were analysed, it was found that a surface layer of peroxide significantly enhanced CuO reducibility in favor of H2 oxidation. Moreover, it seemed to act as an inhibitor to suppress CuO reduction against CO (carbon monoxide) oxidation. This reverse effect of the peroxide on the two oxidation reactions was found to be caused by the modification of the surface sites where the reaction takes place. The scientists were convinced that by making out the bonding sites and learning how they enhanced or inhibited oxidation, they could steer the reaction of the gases. Therefore, they had to get a clearer picture of the reaction dynamics.
The first step was to study the reaction in situ as peroxides are extremely reactive and reactions happen fast. The scientists used in-situ infrared (IR) spectroscopy to get a better understanding of the chemical properties of the material and looked at the way the radiation was absorbed or reflected under reaction conditions. In this manner, they were able to make out different forms of peroxide, with very slight variations in the oxygen they were carrying, which would not have been possible to identify on the metal oxide surface otherwise.
The second technique the team used on the samples was ambient pressure X-ray Photoelectron Spectroscopy (XPS). XPS uses lower-energy x-rays to get electrons out of the sample. The energy of these electrons makes it possible to analyse the chemical properties of atoms in the sample and thus achieve a better catalyst in the long run.
For decades, scientists have tried to improve the functioning of copper oxide catalysts. In 2019, the influence of copper content in the monometallic catalysts supported on the CeO2·Al2O3 binary oxide system was analysed with a view to their catalytic activity and physicochemical properties in the process of oxy-steam reforming of methanol. It was found that activity and selectivity was largely dependent on the content of copper and dispersion on the catalysts surface. The optimal copper content amounted to 20 wt% of Cu. Catalysts with 20 wt% of Cu showed the highest methanol conversion and reaction rate value compared to the rest of the investigated catalysts systems. The kinetic measurements performed in oxy-steam reforming of methanol on 20%Cu/CeO2·Al2O3 catalysts, showed an activation energy for this system equal Ea (OSRM) = 66.56 kJ/mol.
Image: The methanol conversion in the oxy-steam reforming of methanol over Cu/CeO 2 ·Al2O3 catalysts (metal loading = 5, 20, 40 and 60 wt%). Reaction condition: weight of catalyst = 0.1 g, H2O/CH3OH/O2 molar ratio in the reaction mixture = 1/1/0.4, temperature of the reaction 160 and 200 °C. The catalytic teste were performed under atmospheric pressure (GHSV = 26,700 h −1 )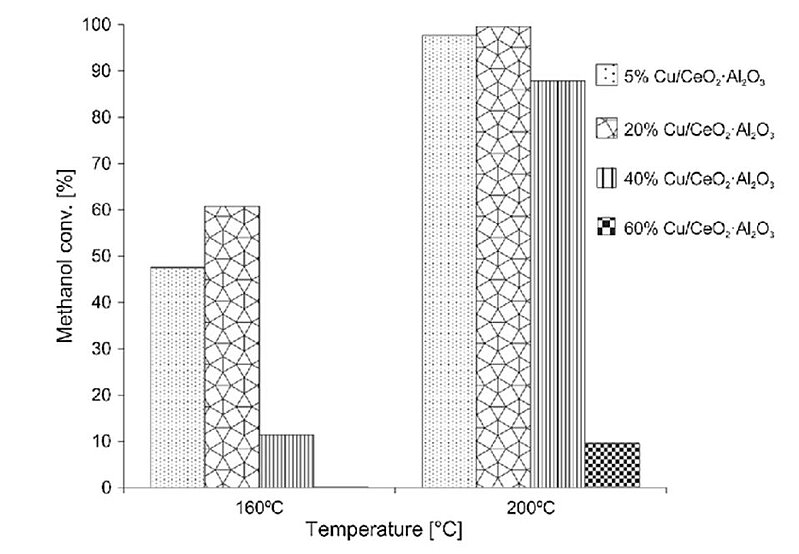
Source: Pawel Mierczynski, Magdalena Mosińska – Kuszyńska, Waldemar Maniukiewicz, Magdalena. Nowosielska/ Oxy-steam reforming of methanol on copper catalysts/ Reaction Kinetics, Mechanisms and Catalysis 127(2), June 2019/ DOI:10.1007/s11144-019-01609-6/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2020, bimetallic Cu-Ni catalysts supported on binary oxides containing ZnO, ZrO2, CeO2 and Al2O3 were analysed in hydrogen production by means of oxidative steam reforming of methanol (OSRM). Their physicochemical properties were studied extensively using various methods such as X-ray diffraction analysis, Scanning electron microscopy (SEM) and Energy dispersive X-ray spectroscopy (EDS), Time-of-Flight Secondary Ion Mass Spectrometry and X-ray photoelectron spectroscopy. The reactivity measurements proved that the active phase and support composition played an important role in the activity of the catalyst in the OSRM. The most active system at higher temperatures was found to consist of 30% Cu - 10% Ni/CeO2·Al2O3, with the Cu0.8Ni0.2 alloy formation stipulating high catalytic activity. Also, the reactivity results showed that the most active catalyst exhibited high acidity and was easily reduced. At low temperatures, 30% Cu - 10% Ni/ZrO2·Al2O3 had the best catalytic properties. The obtained results showed that the use Cu-Ni catalysts for hydrogen production might be an promising method.
Image: SEM images of calcined supports in an air atmosphere at 400°C for 4 h
Source: Magdalena Mosińska – Kuszyńska, Natalia Stępińska, Waldemar Maniukiewicz, Jacek Rogowski/ Hydrogen Production on Cu-Ni Catalysts via the Oxy-Steam Reforming of Methanol/ Catalysts 10(3):273, March 2020/ DOI:10.3390/catal10030273/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
The insights gained from this study may have great bearing on the design of future catalysts: Metal oxides have found wide application as catalysts or components in catalysts. By tuning peroxide formation on other oxides surface reactions other catalytic processes could be blocked or enhanced. The results of this study may apply to other types of reactions and other catalysts besides copper too as the findings and the processes as well as techniques that led to them could find their ways into related research. The successful completion of these experiments can be mainly attributed to the fact that the instruments used were connected. Therefore, the samples could be moved in a controlled environment between these two techniques and studied in situ to get complementary information.
Gaining a better understanding of oxide catalysts gives researchers more control of the chemical reactions they produce, including solutions for clean energy. This may take us one step further to achieving energy-efficient as well as clean processes.
By the Editorial Board
