Solid-state batteries are batteries which use solid electrolyte materials instead of electrochemical gels and liquids to power devices. They have high safety efficiency and energy density as well as function under a wide variety of operating temperatures. Despite their overall good efficiencies, they contain certain elements which may cause a disturbance in the free flow of electrons between the cathode and the ion-conducting layer, which may lead to a loss of energy.
Now (2021), a team of scientists at Sandia Laboratory may have discovered the reason for the impaired efficiency of solid-state batteries and found a way to make them last longer and operate more efficiently using a microscopic method for measuring their electrical potential. They employed Kelvin probe force microscopy, which can measure the electrical potential on a surface, as they knew that it was important for their research to find out where the voltage drops within the battery occurred in order to be able to reduce performance-impairing resistances. They cut the battery in half longitudinally, charged and discharged it and performed measurements over the entire battery. This enabled the team to discover that a fundamental part of the electrical potential of the battery was lost at the boundary between the electrolyte and the anode terminal.
During their research, the team collaborated with researchers at the National Institute of Standards and Technology Center for Neutron Research to confirm their findings. They used a technique called neutron depth profiling which can measure where lithium ions are at a particular moment.
Improving solid-state batteries has been a constant concern of scientific research for many years. In 2019, scientists designed and employed a computational framework to screen a wide range of substances for use as buffer layers between oxide cathodes and sulphide solid electrolytes and anaylsed which were the key factors affecting the stability of the material. They found that Li content and oxygen bonding covalency played an important role in this process. They also found that many of the materials improving efficiencies were polyanionic oxides that could substantially outperform conventional oxide buffers.
In Septmeber 2021, scientists published a paper on a new type of battery that combined two battery sub-fields into a single battery. The battery had both a solid-state electrolyte and an all-silicon anode, making it a silicon all-solid-state battery. In addition, micro-silicon was employed, which has the advantage of being less processed and not as expensive as nano-silicon that is used more often. In addition to removing all carbon and binders from the anode, the team also removed the liquid electrolyte. Instead, they used a sulphide-based solid electrolyte. Their experiments proved this solid electrolyte was extremely stable in batteries with all-silicon anodes. All in all, the study showed that the new battery was safe, long lasting, and energy dense which made it useful for a wide range of applications from grid storage to electric vehicles.
Example: WO2021251598A1. All-solid-state battery comprising silicon (si) as anode active material
Abstract. The present invention relates to an all-solid-state battery comprising silicon (Si) as an anode active material. The all-solid-state battery, according to the present invention, does not include a conductive material and a solid electrolyte in an anode, and uses the minimum amount of a binder. Due to such structural characteristics, the battery according to the present invention has excellent electrochemical characteristics such as heat-resistant stability, energy density, life characteristics, and coulombic efficiency.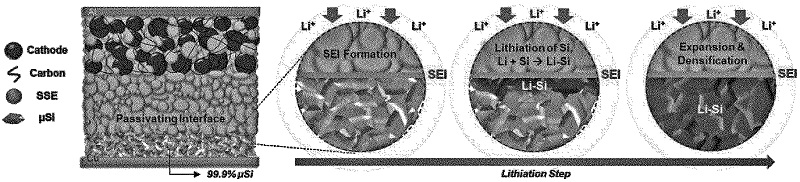
Source: WO2021251598A1
The new insights into the functioning of a battery might have a lot of advantages for future battery design: it might serve as a model that can be used to develop better battery materials. The research is also a big step forward concerning the engineering of interfaces to improve ion flow. Therefore, it could also have a tremendous impact on improving the speed of lithium ions in Si anodes.
The next step in the research will be to use their newly-developed technique to analyse other batteries and solid-state electrical systems, like the electrochemical random access memory invented at Sandia. The scientists hope that in the long run they can make a valuable contribution to enhancing battery-powered devices.
