Aenert. Research Laboratory news
In today’s industrialised world, hydrogen plays an important role in many areas including agriculture and industry. Hydrogen has the potential to reduce carbon dioxide emissions as neither its production nor its combustion release CO2 emissions into the atmosphere. Moreover, it can also be transformed into electricity or synthetic gas. However, to date it is still mostly generated from fossil fuels. Fossil fuels are among the main culprits when it comes to CO2 emissions and can therefore aggravate the problem of climate change. For this reason, scientists are relentlessly searching for means to harness the power of hydrogen without creating harmful substances.
Now (2023), scientists at Tel Aviv University achieved a major breakthrough in the production of sustainable hydrogen as their method not only avoids air pollution but has also proved to be highly efficient.
In plants, it is usually the sun which powers the plant enzymes that decompose the water molecules into gases. This is called hydrogenase. The Israeli scientists now developed a method where the sun's power could be easily replaced by electricity. The only problem was that they had to find a solution to the problem of the enzymes being naturally repelled by the electric charge. So, the team of scientists created a chemical treatment which could prevent this reaction: a hydrogel mixed with hydrogenase.
The hydrogel was used to attach the enzyme to the electrode and thus hydrogen could be produced with the help of a biocatalyst and over 90 percent efficiency which means that 90 percent of the electrons introduced into the system were deposited in the hydrogen without any secondary processes.
The great achievement of this study was that they took a known substance, the hydrogel, and repurposed it for the production of hydrogen. They soaked the electrode in the gel, which contained the enzymes for producing hydrogen. The gel was found to be able to hold the enzymes for a long time, even under electric voltage, and thus enabled uninhibited hydrogen production. The gel formed nanometric fibers when put into water which were able to stick the enzyme to the electrode which prevented the enzymes being repelled by the electric charge. The gel was also tested with two other enzymes, in addition to the hydrogenase, and proved that it was able to attach different enzymes to the electrode.
Image: Electrochemical properties of HydA encapsulated in an FmocFF-soaked electrode. (A) Proposed scheme of the electrochemical activity at different potentials. Yellow triangles represent MVox, blue triangles represent MVred, HydA is represented by its protein crystallographic structure, light blue circles represent H2, and the dark-gray and light-yellow areas, respectively, represent the carbon felt electrode soaked with the FmocFF hydrogel. I. Reduction of MV by the electrode and shuttling of electrons to HydA, which catalyzes H2 production. II. Oxidation of the MV pool at the electrode. III. H2 oxidation by HydA reduces MV, which shuttles the electrons back to the electrode. (B) Cyclic voltammogram of the FmocFF hydrogel soaked on a carbon felt electrode supplemented with HydA and MV. (C) Accumulated H2 produced overnight (O.N.) by the electrochemical assay versus the amount of enzyme loaded on the working electrode in the FmocFF hydrogel (red circles), the FmocLL hydrogel (blue tringles), and the solution (black diamonds). Error bars represent mean ± SD of at least six independent experiments. (D–G) Corresponding chronoamperometries of O.N. electrochemical assays of FmocFF-, FmocLL-, and solution-soaked electrodes (red, blue, and black, respectively)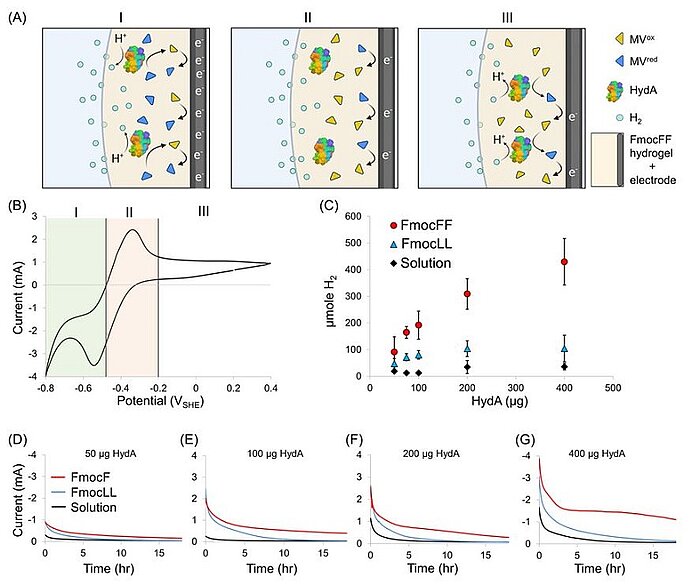
Source: Itzhak Grinberg, Oren Ben-Zvi, Lihi Adler-Abramovich, Iftach Yacoby/ Peptide self-assembly as a strategy for facile immobilization of redox enzymes on carbon electrodes/ Carbon Energy, 11 July 2023/ doi.org/10.1002/cey2.411/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
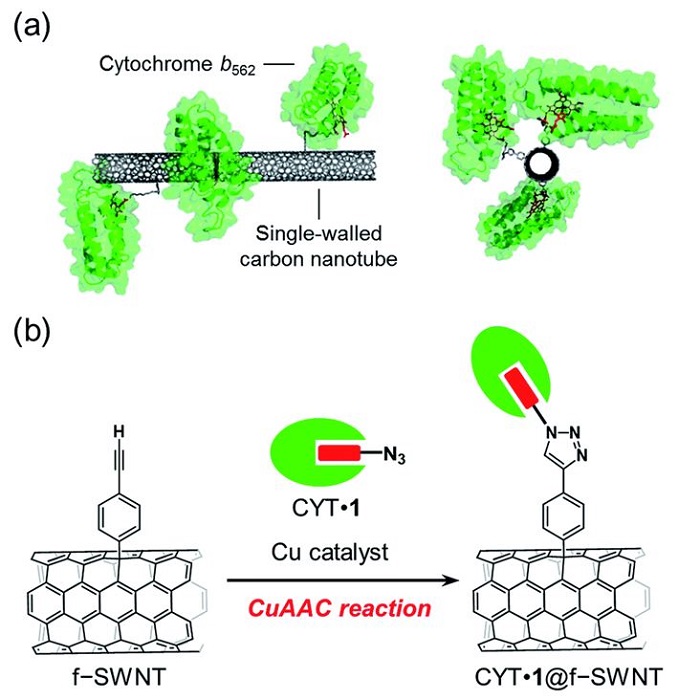
Scientists have long tried to find means to produce hydrogen efficiently and inexpensively. In 2016, for example, scientists researched specifically oriented covalent immobilization of azide-linked cytochrome b562 (CYT) on the sidewall of single-walled nanotubes using the CuAAC reaction, a click chemistry reaction that uses efficient and reliable reactions, such as Cu(I)-catalyzed azide–alkyne cycloaddition, to bind two molecular building blocks. The main benefit of this method using a replaceable heme tethered to an azide moiety was the wide range of applications for functionalisation of wild-type hemoproteins. This method enabled development of oriented redox-active hemoprotein single-walled nanotubes hybrid materials. The method for fabricating hemoprotein–carbon nanomaterial electrodes was shown to have great potential for use in preparing specifically-designed bioelectrode interfaces.
Image: (a) SWNT with covalently-linked cytochrome b562 and (b) the preparation scheme using a copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction
Source: Akira Onoda, Nozomu Inoue, Stéphane Campidelli and Takashi Hayashi/ Cofactor-specific covalent anchoring of cytochrome b562 on a single-walled carbon nanotube by click chemistry/ RSC Advances, Issue 70, 2016, 04 Jul 2016 / DOI doi.org/10.1039/C6RA14195A/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2020, scientists investigated the pH dependence of the redox behaviour of [4Fe–4S]H. They used infrared (IR) spectroelectrochemistry with two different [FeFe] hydrogenases: CrHydA1 from Chlamydomonas reinhardtii, a type of algae, and CpHydA1 from Clostridium pasteurianum, an anaerobic bacterium. They found that, under our experimental conditions, the redox potential of [4Fe–4S]H was independent of pH around physiological values for both enzymes. The redox anticooperativity behaviour between [4Fe–4S]H and the accessory [4Fe–4S] clusters (F-clusters) in CpHydA1, which could modify catalysis at the active site, were also researched. The results showed that in the catalytic cycle of [FeFe] hydrogenases no protonation at or near [4Fe–4S]H happened, but instead a process took place in which protonation of [2Fe]H dove catalysis.
Image: Structure and catalytic cycle of [FeFe] hydrogenase. (A) Structure of the H-cluster. (B) Structure of CrHydA1 apoprotein (PDB ID 3LX4) (14) with the H-cluster modeled from the CpHydA1 structure (PDB ID 4XDC). (15) The H-cluster is composed of the [4Fe–4S]H and [2Fe]H subclusters and is shown in a ball-and-stick representation, along with the cysteine ligating [4Fe–4S]H. (C) Structure of CpHydA1 artificially maturated with the diiron ADT cofactor (PDB ID 4XDC). (15) The H-cluster and accessory F-clusters are shown as balls and sticks, and the protein backbone is shown as a cartoon. The H-domain harboring the H-cluster in both enzymes is colored blue, and the F-domain containing the accessory F-clusters, only present in CpHydA1, is colored pink. (D) Simple form of Model 1 proposed for the catalytic cycle, where [4Fe–4S]H is represented by a diamond and [2Fe]H is represented as a rectangle. The CN– and terminal CO ligands are omitted for clarity. The H-cluster containing the propane 1,3-dithiolate (PDT) ligand with methylene in the bridgehead only cycles between Hox and Hred, while the H-cluster containing ADT participates in the full cycle. A recent work of Lorent and coworkers has uncovered additional forms of the Hhyd state. (16) (E) Model 2 of the catalytic cycle (2) where the Hox state becomes reduced and protonated on [4Fe–4S]H simultaneously to give Hred’. Hred’ is then reduced and protonated simultaneously to give Hhyd, where the proton on [4Fe–4S]H is retained. Hhyd then acquires an additional proton, reacting to make H2, leaving a proton on [4Fe–4S]H in the HoxH state. The Hred and Hsred states are inactive, bridging hydride containing states. Hred’ can also be protonated at an alternative site near [4Fe–4S]H to give the Hred’H state. Hred’H is also known to be the reduced and protonated form of HoxH. In this model, the H-cluster containing the ADT ligand can participate in the full cycle, while the PDT containing H-cluster can only access the Hox, Hred’, HoxH, and Hred’H states. Cycle 2 was adapted from three related models appearing in previous works, while trying to fit Hred’H into its most logical location. (2,3,17)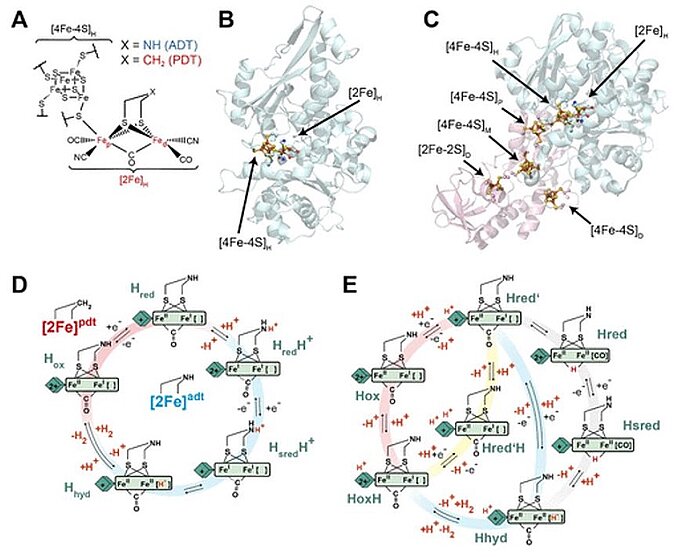
Source: Patricia Rodríguez-Maciá, Nina Breuer, Serena DeBeer, James A. Birrell/ Insight into the Redox Behavior of the [4Fe–4S] Subcluster in [FeFe] Hydrogenases/ ACS Catal. 2020, 10, 21, 13084–13095, October 27, 2020/ doi.org/10.1021/acscatal.0c02771/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
There are several benefits involved in using a hydrogel for hydrogen production: The gel can retain the enzyme for a long time, even under the electric voltage, and enables hydrogen production with great efficiency and at more inexpensive conditions, for example, in salt water. Another advantage is that the gel assembles itself when you put the material in water, and it settles into nanometric fibers that form the gel. Moreover, under laboratory conditions, the enzymes were electrified by an electrode. As a result, there is no demand for extreme conditions. Electricity could be utilized from renewable sources such as solar panels or wind turbines. However, the enzyme tries to avoid the electric charge, so it needs to be held in place through chemical treatment. But the scientists found a simple and efficient way to attach the enzyme to the electrode and utilize it.
Nowadays, environmentally-friendly hydrogen is generated primarily through electrolysis, where precious and rare metals such as platinum along with water distillation are needed to make the process work. This makes the green hydrogen production almost 15 times more expensive than the less sustainable ‘grey’ one. The scientists hope that in the future, it will be possible to use this method in large-scale settings, to lower costs and enable large-scale use of green hydrogen in industry, agriculture, and as a clean energy source.
By the Editorial Board