Flow batteries consist of electrochemical cells containing chemical components which are dissolved in liquids and pumped through the system on separate sides of a membrane to provide the chemical energy needed. The ion exchange takes place through the membrane while both liquids remain in their respective spaces. Cell voltage ranges from 1.0 to 2.43 volts. Flow batteries can be used like fuel cells or rechargeable batteries. While they have technical advantages over conventional rechargeables, such as potentially separable liquid tanks and incredible longevity, current installations are comparatively less powerful and require more sophisticated electronics.
Now (2021), Chinese scientists have designed a molecular compound that can be used as a low-cost electrolyte for a stable flow battery. This battery can retain 99.98% capacity per cycle. It has two tanks of opposing liquid electrolytes where the battery pumps the positive and negative liquids along a membrane separator wedged between electrodes, which facilitates ion exchanges to produce energy. Significant work has been dedicated to developing the negative electrolyte liquid. The scientists focused their attention on TEMPO (tetramethylpiperidinoxyl), a chemical compound with easily reversed oxidation states and high energy potential, which positive electrolytes need to work more efficiently. However, TEMPO cannot be directly applied to aqueous redox flow batteries due to the high hydrophobicity of the molecular makeup as when unmodified it will not dissolve in the liquid which is essential for facilitating the energy exchange in the flow batteries. Therefore, they developed a method to functionalise TEMPO with viologen, an organic compound that causes highly reversible redox reactions, to improve hydrophilicity of TEMPO since viologen is highly soluble in water and thus also increases TEMPO’s ability to dissolve in water. Viologen can also chemically withdraw electrons from atomic partners, which elevates its potential to change its oxidative state. Moreover, viologen is a salt, which endows TEMPO with a decent conductivity in an aqueous solution.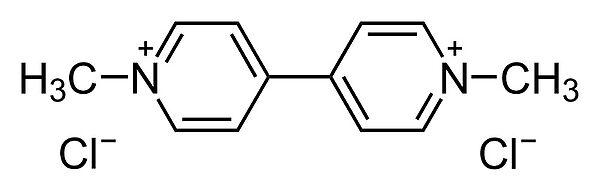
Chemical structure of paraquat (prominent viologen). Calvero. Public Domain
The design of aqueous flow batteries has been at the centre of scientific attention for several years. In 2014, scientists designed a novel Organic Redox Flow Battery (ORBAT) for large-scale energy storage. For this battery two different water-soluble organic redox couples, quinones, on the positive and negative side of a flow battery were used. No precious metal catalyst was needed because of the fast proton-coupled electron transfer processes. Furthermore, in acid media, the quinones exhibited good chemical stability. The battery could be charged and discharged multiple times at high faradaic efficiency without any noticeable degradation of performance.
In 2017, an aqueous flow battery based on low-cost, non-flammable, noncorrosive, and earth-abundant elements was designed. During charging, electrons were stored in a concentrated water solution, which was able to rapidly incorporate electrons without the assistance of any metal electrocatalyst. The electrons were withdrawn from a second water solution of a food additive, potassium ferrocyanide. The flow battery showed a cell potential of 1.21 V and a coulombic efficiency exceeding 99%. Also, various molecular modifications involving substitution for hydrogens on the aryl ring were put in place to block decomposition. This increased capacity retention rates of up to 99.96%.
The new battery design using TEMPO and viologen has several advantages: it was able to significantly improve the efficiency of flow batteries. It can overcome all the disadvantages of TEMPO as sole electrolyte by adding viologen and realises its application in aqueous redox flow battery. When the synthesized viologen-modified TEMPO was tested in a flow battery, the researchers found that the battery retained a capacity of 99.98% per cycle, meaning the battery could hold nearly all its stored energy when not in active use. The molecular design concept provides a strategy for novel organic electroactive materials and lays a foundation for the application of aqueous organic flow battery.
The research results have great potential for further application of enhanced TEMPO in aqueous flow batteries. The inclusion of viologen into the electrolyte material was shown to increase TEMPO’s aqueous solubility, elevate the redox potential and prevent its crossover, which will eventually lead to the development of energy-dense, stable, and lost-cost aqueous redox flow batteries.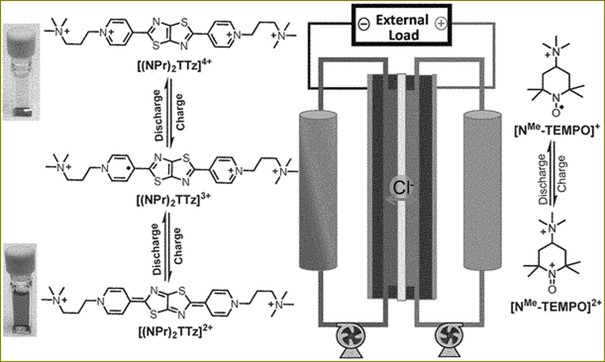
Source: Applications of low-cost, thermal and electrochemically stable organic compounds as high performance redox active materials in redox flow batteries. US20200168910A1