Aenert. Research Laboratory news
Plant biomass is a renewable, abundant, and eco-friendly source of energy. Using plant biomass can reduce reliance on fossil fuels and improve the problem of environmental pollution. A variety of biofuels such as biodiesel, biohydrogen, bioethanol, biogas stand alongside their fossil counterparts nowadays and are produced through different production processes. Their production depends on enzymes either for pretreatment of feedstock or enzymes play a vital role in synthesis process itself. How well these enzymes interact with the feedstock has a great bearing on process efficiency as well as feasibility. Therefore, commercialization of biofuels from variable biomass has certain limitations related to the enzymes involved in the production.
Plant biomass contains energy-rich complex sugar molecules which are generated from photosynthesis. A rigid cell wall made of sugars surrounds the plant cells as well as a material called lignin providing structural support. Much as lignin is needed to provide a healthy plant, it needs to be reduced if the sugars are to be harnessed and converted into fuels. This has been the focus of research aimed at using plants to generate fuels and other products commonly made from petroleum.
Now (2023), scientists at Brookhaven National Laboratory have created enzymes to modify grass plants so that their biomass content can be efficiently tuned into biofuels and other bioproducts. These enzymes can modify molecules contained in plant cell walls to provide access to fuel-generating sugars normally locked within complex structures.
For nearly 15 years, the team of scientists has been working on this problem using engineered enzymes called monolignol 4-O-methyltransferases (MOMTs). These synthetic enzymes can change the chemical structure of the main building blocks of lignin, monolignols. This prevents them from linking together, which reduces the lignin content of plants and makes the sugars more accessible.
In prior work, the scientists successfully expressed MOMTs in poplar trees. These enzymes showed great ability to reduce the lignin content of the trees and enable increased sugar release from the plants. In the recent study, the potential applications of the MOMT enzymes in grass plants was looked at. Grasses other than trees can grow in difficult environments, such as soils deficient in water or nutrients. If engineered plants were to be grown in such environments, this could potentially produce biomass optimized for conversion to fuel and bioproducts which does not have to be grown on land needed to produce food crops.
In this study, the team of scientists focused on two versions of the enzyme, MOMT4 and MOMT9, each designed to modify a different lignin subunit. Collaborating with researchers from Kyoto University in Japan, they carried out chemical analyses on rice plants engineered to express either MOMT4 or MOMT9. The research proved that there was less lignin in the modified grass plants compared to unaltered plants.
However, even though MOMT4 and MOMT9 were specifically designed to act upon monolignols, the test results revealed that these engineered enzymes also acted on other components as well. Both MOMTs influenced the cross-linking phenolics and also a phenolic called tricin, a lignin precursor only found in grass plants. As the structures of the cross-linking phenolics and tricin were changed, the MOMTs also reduced the incorporation of those compounds into the cell walls which further weakened them. Modified phenolics were also found to have accumulated in the rest of the plant tissue. Moreover, plants having MOMT9 did not grow as tall as the unaltered plants, which reduced the quantity of biomass from which sugar could be accessed. The plants also could not to produce seeds, which means that altered plants cannot reproduce. To tackle these problems, future research will focus on methods for controlling how lignin gets modified in different parts of the plant.
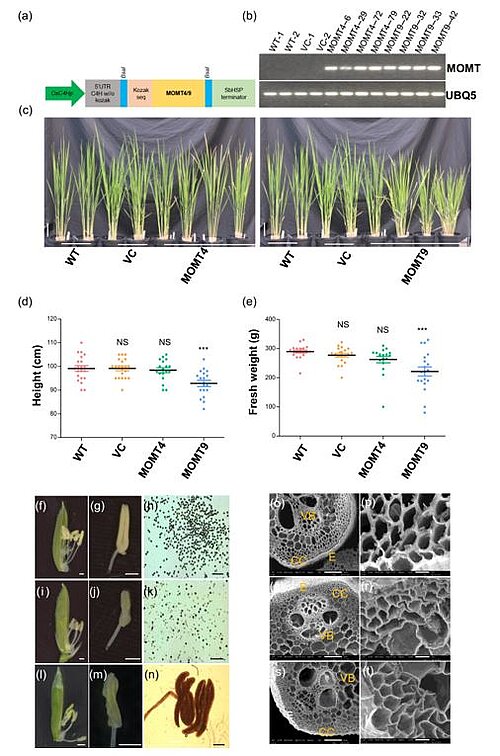
Image: Phenotypic analysis of MOMT4 and MOMT9 transgenic plants. (a) Expression cassette of MOMT4/9 under OsC4H promoter with OsC4H 5′ untranslated region, a kozak sequence and a sorghum heat shock protein gene terminator (SbHSP). (b) The RT-PCR analysis of MOMT4/9 transgene expression in the WT, empty VC, MOMT4, or MOMT9 overexpression lines. Rice ubiquitin 5 gene (UBQ5) was used as the control. (c) Morphology of the regenerated 1.5-month-old MOMT4 and MOMT9 overexpression plants in T0 generation after first time cutting. Scale bar = 10 cm. (d and e) The measurements of plant height (d) and aerial biomass yield (e). Data are presented as mean ± s.e. (n = 20). Each data point represents individual MOMT4 or MOMT9 transgenic line. *** Indicates statistically significant difference with P <0.001, compared to the WT (one-way ANOVA test; Tukey's multiple-comparison test). (f–n) Floret and anther morphology, and Iodine-potassium iodide staining of mature pollen grains of the WT (f–h), MOMT4 (i–k), and MOMT9 (l–n) overexpression plants. Scale bar = 500 μm in (f–n). Note that MOMT9 anthers did not release any viable pollen grains (n). (o–t) Scanning electron micrographs of transverse sections of the fourth stem internodes of T0 generation plants after the fourth successive asexual propagation–regeneration, and the enlarged vision of their collenchyma cells of theWT (o, p), MOMT4 (q, r), and MOMT9 (s, t). E, epidermis; CC, collenchyma cells; VB, vascular bundle. Scale bar = 50 μm in (o, q, s) and 5 um in (p, r, t)
Source: Nidhi Dwivedi, Senri Yamamoto, Yunjun Zhao, Guichuan Hou, Forrest Bowling, Yuki Tobimatsu, Chang-Jun Liu/ Simultaneous suppression of lignin, tricin and wall-bound phenolic biosynthesis via the expression of monolignol 4-O-methyltransferases in rice/ Plant Biotechnology Journal, 05 October 2023/ doi.org/10.1111/pbi.14186/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International
Finding effective means of biofuel production is an important task if we are to mitigate the harmful effects of climate change. In 2015, scientists tested if specific remodeling the active site of a monolignol 4-O-methyltransferase would yield an enzyme that specifically methylated the condensed guaiacyl lignin precursor coniferyl alcohol. Through combining crystal structural information with combinatorial active site saturation mutagenesis and using the engineered promiscuous enzyme, MOMT5 (T133L/E165I/F175I/F166W/H169F), they remodeled the substrate binding pocket by the addition of four substitutions, i.e. M26H, S30R, V33S, and T319M, which created a mutant enzyme capable of discriminately etherifying the para-hydroxyl of coniferyl alcohol. The engineered enzyme variant substantially reduced substrate binding pocket imposing a steric hindrance. The resulting enzyme variant was found to be excellent for modulating lignin composition and/or structure in planta.
Image: The scheme of lignin polymerization process and MOMT-catalyzed reaction. A, lignin monomeric precursors. B, MOMT-catalyzed methylation reaction. C, the dehydrogenation of monolignols. D, the subsequent polymerization process

Source: Yuanheng Cai, Mohammad-Wadud Bhuiya, John Shanklin, Chang-Jun Liu/ Engineering a Monolignol 4-O-Methyltransferase with High Selectivity for the Condensed Lignin Precursor Coniferyl Alcohol*/ Plant Biology| Volume 290, ISSUE 44, October 2015/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2016, scientists expressed an engineered 4-O-methyltransferase capable of chemically modifying the phenolic moiety of lignin monomeric precursors and thus preventing their incorporation into the lignin polymer. This substantially changed the lignin content and structure of the hybrid aspens. Woody biomass from the transgenic aspens exhibited a 62% increase in the release of simple sugars and up to a 49% increase in the yield of ethanol when subjected to enzymatic digestion and yeast-mediated fermentation. Also, the cell wall structural changes were found to not inhibit growth and biomass production of the trees.
Image: (a) Three-month-old hybrid aspens of control (left) and three MOMT4 independent transgenic lines (right). (b,c) Phloroglucinol-HCl staining of the stem cross-sections of control (b) and MOMT4-0 transgenic line (c). (d,e) Mäule staining of the stem cross-sections of control (d) and MOMT4-0 transgenics (e). Scale bars, 1 mm. (f) Acetyl bromide total lignin content in the cell walls of control and MOMT4 transgenic aspen stems. (g) The monomers released by thioacidolysis from the stem cell walls of MOMT4 transgenic aspens; S, syringyl; G, guaiacyl; H, p-hydroxyphenyl; CWR, cell wall residues; Ctrl., control. Data in f,g represent mean±s.e. with three biological replicates (each with three technical repeats) for the control and three technical repeats for the individual transgenic lines. ** Indicates significant difference of lignin content (f) or S-monomer (g) compared to the control with P<0.01 (Student’s t-test)

Source: Yuanheng Cai, Kewei Zhang, Hoon Kim, Guichuan Hou, Xuebin Zhang, Huijun Yang, Huan Feng, Lisa Miller, John Ralph & Chang-Jun Liu/ Enhancing digestibility and ethanol yield of Populus wood via expression of an engineered monolignol 4-O-methyltransferase/ Nature Communications volume 7, Article number: 11989 (2016), 28 June 2016/ doi.org/10.1038/ncomms11989/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
Reducing the lignin content has several advantages: With lesser lignin in the cell walls, up to 30% more sugar could be collected from plants expressing MOMT4 and up to 15% more sugar in plants expressing MOMT9, compared to unaltered plants. Through fermentation, sugar biofuels like ethanol can be produced through conversion. Ethanol is a common additive used to lower the fossil fuel content of gasoline.
The next steps will include analysing if their MOMT enzymes can optimise sugar yields from other grass plant species. The scientists are hopeful that the enzyme can be used to modify grass energy crops like sorghum and bamboo. They are also convinced that it will help overcome some of the waste that occurs with unmodified biomass crops.
By the Editorial Board
Plant biomass is a renewable, abundant, and eco-friendly source of energy. Using plant biomass can reduce reliance on fossil fuels and improve the problem of environmental pollution. A variety of biofuels such as biodiesel, biohydrogen, bioethanol, biogas stand alongside their fossil counterparts nowadays and are produced through different production processes. Their production depends on enzymes either for pretreatment of feedstock or enzymes play a vital role in synthesis process itself. How well these enzymes interact with the feedstock has a great bearing on process efficiency as well as feasibility. Therefore, commercialization of biofuels from variable biomass has certain limitations related to the enzymes involved in the production.
Plant biomass contains energy-rich complex sugar molecules which are generated from photosynthesis. A rigid cell wall made of sugars surrounds the plant cells as well as a material called lignin providing structural support. Much as lignin is needed to provide a healthy plant, it needs to be reduced if the sugars are to be harnessed and converted into fuels. This has been the focus of research aimed at using plants to generate fuels and other products commonly made from petroleum.
Now (2023), scientists at Brookhaven National Laboratory have created enzymes to modify grass plants so that their biomass content can be efficiently tuned into biofuels and other bioproducts. These enzymes can modify molecules contained in plant cell walls to provide access to fuel-generating sugars normally locked within complex structures.
For nearly 15 years, the team of scientists has been working on this problem using engineered enzymes called monolignol 4-O-methyltransferases (MOMTs). These synthetic enzymes can change the chemical structure of the main building blocks of lignin, monolignols. This prevents them from linking together, which reduces the lignin content of plants and makes the sugars more accessible.
In prior work, the scientists successfully expressed MOMTs in poplar trees. These enzymes showed great ability to reduce the lignin content of the trees and enable increased sugar release from the plants. In the recent study, the potential applications of the MOMT enzymes in grass plants was looked at. Grasses other than trees can grow in difficult environments, such as soils deficient in water or nutrients. If engineered plants were to be grown in such environments, this could potentially produce biomass optimized for conversion to fuel and bioproducts which does not have to be grown on land needed to produce food crops.
In this study, the team of scientists focused on two versions of the enzyme, MOMT4 and MOMT9, each designed to modify a different lignin subunit. Collaborating with researchers from Kyoto University in Japan, they carried out chemical analyses on rice plants engineered to express either MOMT4 or MOMT9. The research proved that there was less lignin in the modified grass plants compared to unaltered plants.
However, even though MOMT4 and MOMT9 were specifically designed to act upon monolignols, the test results revealed that these engineered enzymes also acted on other components as well. Both MOMTs influenced the cross-linking phenolics and also a phenolic called tricin, a lignin precursor only found in grass plants. As the structures of the cross-linking phenolics and tricin were changed, the MOMTs also reduced the incorporation of those compounds into the cell walls which further weakened them. Modified phenolics were also found to have accumulated in the rest of the plant tissue. Moreover, plants having MOMT9 did not grow as tall as the unaltered plants, which reduced the quantity of biomass from which sugar could be accessed. The plants also could not to produce seeds, which means that altered plants cannot reproduce. To tackle these problems, future research will focus on methods for controlling how lignin gets modified in different parts of the plant.
Image: Phenotypic analysis of MOMT4 and MOMT9 transgenic plants. (a) Expression cassette of MOMT4/9 under OsC4H promoter with OsC4H 5′ untranslated region, a kozak sequence and a sorghum heat shock protein gene terminator (SbHSP). (b) The RT-PCR analysis of MOMT4/9 transgene expression in the WT, empty VC, MOMT4, or MOMT9 overexpression lines. Rice ubiquitin 5 gene (UBQ5) was used as the control. (c) Morphology of the regenerated 1.5-month-old MOMT4 and MOMT9 overexpression plants in T0 generation after first time cutting. Scale bar = 10 cm. (d and e) The measurements of plant height (d) and aerial biomass yield (e). Data are presented as mean ± s.e. (n = 20). Each data point represents individual MOMT4 or MOMT9 transgenic line. *** Indicates statistically significant difference with P <0.001, compared to the WT (one-way ANOVA test; Tukey's multiple-comparison test). (f–n) Floret and anther morphology, and Iodine-potassium iodide staining of mature pollen grains of the WT (f–h), MOMT4 (i–k), and MOMT9 (l–n) overexpression plants. Scale bar = 500 μm in (f–n). Note that MOMT9 anthers did not release any viable pollen grains (n). (o–t) Scanning electron micrographs of transverse sections of the fourth stem internodes of T0 generation plants after the fourth successive asexual propagation–regeneration, and the enlarged vision of their collenchyma cells of theWT (o, p), MOMT4 (q, r), and MOMT9 (s, t). E, epidermis; CC, collenchyma cells; VB, vascular bundle. Scale bar = 50 μm in (o, q, s) and 5 um in (p, r, t)

Source: Nidhi Dwivedi, Senri Yamamoto, Yunjun Zhao, Guichuan Hou, Forrest Bowling, Yuki Tobimatsu, Chang-Jun Liu/ Simultaneous suppression of lignin, tricin and wall-bound phenolic biosynthesis via the expression of monolignol 4-O-methyltransferases in rice/ Plant Biotechnology Journal, 05 October 2023/ doi.org/10.1111/pbi.14186/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International
Finding effective means of biofuel production is an important task if we are to mitigate the harmful effects of climate change. In 2015, scientists tested if specific remodeling the active site of a monolignol 4-O-methyltransferase would yield an enzyme that specifically methylated the condensed guaiacyl lignin precursor coniferyl alcohol. Through combining crystal structural information with combinatorial active site saturation mutagenesis and using the engineered promiscuous enzyme, MOMT5 (T133L/E165I/F175I/F166W/H169F), they remodeled the substrate binding pocket by the addition of four substitutions, i.e. M26H, S30R, V33S, and T319M, which created a mutant enzyme capable of discriminately etherifying the para-hydroxyl of coniferyl alcohol. The engineered enzyme variant substantially reduced substrate binding pocket imposing a steric hindrance. The resulting enzyme variant was found to be excellent for modulating lignin composition and/or structure in planta.
Image: The scheme of lignin polymerization process and MOMT-catalyzed reaction. A, lignin monomeric precursors. B, MOMT-catalyzed methylation reaction. C, the dehydrogenation of monolignols. D, the subsequent polymerization process

Source: Yuanheng Cai, Mohammad-Wadud Bhuiya, John Shanklin, Chang-Jun Liu/ Engineering a Monolignol 4-O-Methyltransferase with High Selectivity for the Condensed Lignin Precursor Coniferyl Alcohol*/ Plant Biology| Volume 290, ISSUE 44, October 2015/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2016, scientists expressed an engineered 4-O-methyltransferase capable of chemically modifying the phenolic moiety of lignin monomeric precursors and thus preventing their incorporation into the lignin polymer. This substantially changed the lignin content and structure of the hybrid aspens. Woody biomass from the transgenic aspens exhibited a 62% increase in the release of simple sugars and up to a 49% increase in the yield of ethanol when subjected to enzymatic digestion and yeast-mediated fermentation. Also, the cell wall structural changes were found to not inhibit growth and biomass production of the trees.
Image: (a) Three-month-old hybrid aspens of control (left) and three MOMT4 independent transgenic lines (right). (b,c) Phloroglucinol-HCl staining of the stem cross-sections of control (b) and MOMT4-0 transgenic line (c). (d,e) Mäule staining of the stem cross-sections of control (d) and MOMT4-0 transgenics (e). Scale bars, 1 mm. (f) Acetyl bromide total lignin content in the cell walls of control and MOMT4 transgenic aspen stems. (g) The monomers released by thioacidolysis from the stem cell walls of MOMT4 transgenic aspens; S, syringyl; G, guaiacyl; H, p-hydroxyphenyl; CWR, cell wall residues; Ctrl., control. Data in f,g represent mean±s.e. with three biological replicates (each with three technical repeats) for the control and three technical repeats for the individual transgenic lines. ** Indicates significant difference of lignin content (f) or S-monomer (g) compared to the control with P<0.01 (Student’s t-test)

Source: Yuanheng Cai, Kewei Zhang, Hoon Kim, Guichuan Hou, Xuebin Zhang, Huijun Yang, Huan Feng, Lisa Miller, John Ralph & Chang-Jun Liu/ Enhancing digestibility and ethanol yield of Populus wood via expression of an engineered monolignol 4-O-methyltransferase/ Nature Communications volume 7, Article number: 11989 (2016), 28 June 2016/ doi.org/10.1038/ncomms11989/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
Reducing the lignin content has several advantages: With lesser lignin in the cell walls, up to 30% more sugar could be collected from plants expressing MOMT4 and up to 15% more sugar in plants expressing MOMT9, compared to unaltered plants. Through fermentation, sugar biofuels like ethanol can be produced through conversion. Ethanol is a common additive used to lower the fossil fuel content of gasoline.
The next steps will include analysing if their MOMT enzymes can optimise sugar yields from other grass plant species. The scientists are hopeful that the enzyme can be used to modify grass energy crops like sorghum and bamboo. They are also convinced that it will help overcome some of the waste that occurs with unmodified biomass crops.
By the Editorial Board
