Aenert. Research Laboratory news
In the light of growing energy demands and climate change, harnessing energy from different sources of energy will become increasingly important. Among these alternative methods of energy production, electrochemical processes like water electrolysis stand out as, on the one hand, they draw on electricity, not on chemical substances and, on the other, can respond to fluctuations in the power supply by connecting electrochemical processes in case of excess electricity or by switching them off in case of an electricity shortage. Water electrolysis generates hydrogen which is a flexible source of energy and plays an important role in any energy scenario revolving around the energy transition.
Now (2023), Fraunhofer IMM is building electrochemical microreactors, also known as “flow cells”, which can be used in green synthesis processes like electrosynthesis. The reactors play an important part in developing and screening different synthesis processes on a laboratory scale so that the synthesis can then be transferred to pilot scale. In cooperation with Heidelberg-based hte GmbH, Fraunhofer IMM has developed a modular and flexible concept for an electrochemical flow cell that they have put to use for high-throughput screening tasks in electrocatalysis.
The basic reactor concept applies a plate stack design. A single electrochemical cell consists of a set of electrode plates and other components. The stack is composed of either one cell for individual operation, or a set of cells that can be operated in parallel, serially or both. The parallel operation mode is especially suited for screening tasks. Thus, studying the influence of the membrane material on the electrochemical process is easy since different membranes can be installed in each of the electrochemical cells in the stack, which are otherwise the same. As a result, the best membrane for the process can be determined and used for the process. The reactor allows for a vast number of cell variations, which means that different membrane materials and electrocatalysts can be modified and tested, for example.
An initial screening module prototype with four electrochemical cells arranged in parallel has been constructed and is currently undergoing validation. If successful, this model can be expanded to 16 parallel cells. The numerous reactor configurations make it possible to adapt the screening platform to different areas of application. As such, other tasks can also be considered in addition to water electrolysis, such as the production of active pharmaceutical ingredients or the decomposition of waste in wastewater treatment.
Scientists have been exploring different means of environmentally-friendly hydrogen production for several years. In 2011, a electro-chemical microreactor was developed in which electrochemical reactions can be carried out in flow. It had two aluminium bodies (50 mm diameter, 25 mm height). The electrodes were made using two PTFE plates (35 mm diameter, 4 mm height) with 0.1 mm platinum foil electrodes attached. The wires for connection to the potentiostat were applied inside the PFTE plate under the platinum. A small gap between the electrodes to avoid the necessity of electrolytes and a simple setup were used in the design. The electrodes were separated by a FEP (fluorinated ethylene propylene) foil of variable thick-ness, into which a rectangular reaction channel was cut (3 × 30 mm), giving an overall channel volume of 23 µL (FEP foil 254 µm thick), and the whole device is held together by steel screws and wing nuts. During the reaction, diaryliodonium compounds were synthesised after performing some initial test reactions.
Known electrochemical reactions were used as test reactions for the electrochemical microreactor. The oxidative methoxylation of furan 4 in methanol with a product obtained in an approximate 1:1 syn:anti ratio showed that our system functioned satisfactorily for electrochemical reactions. Full conversion was achieved as established by GC/MS.
Image: Electrochemical microreactor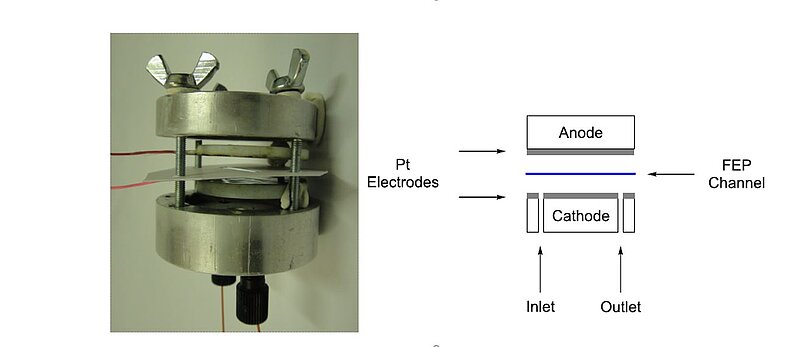
Source: Kevin Watts, William Gattrell, Thomas Wirth/ A practical microreactor for electrochemistry in flow/ Beilstein Journal of Organic Chemistry 7(1):1108-14, August 2011/ DOI:10.3762/bjoc.7.127/ Open Access This is an Open Access article is distributed under the terms of the Attribution 2.0 Generic (CC BY 2.0)
In 2023, scientists reported co-electrolysis of seawater and carbon dioxide (CO2) gas in a solar cell-integrated membraneless microfluidic reactor for continuous synthesis of organic products. The reactor was constructed using polydimethylsiloxane substrate consisting of a central microchannel with a pair of inlets for injection of CO2 gas and seawater and an outlet for removal of organic products. A pair of copper electrodes were installed in microchannel to ensure immediate interaction with incoming CO2 gas and seawater passing through the microchannel. The coupling of solar cell panels with electrodes produced a high-intensity electrical field across the electrodes at low voltage causing the co-electrolysis of CO2 and seawater. The paired electrolysis of CO2 gas and seawater yielded a range of industrially important organics under influence of solar cell-mediated external electric field. The sythesised organic compounds were collected downstream and identified using characterisation techniques. Furthermore, the probable underlying electrochemical reaction mechanisms near the electrodes were analysed for synthesis of organic products. The inclusion of greenhouse CO2 gas as reactant, seawater as electrolyte, and solar energy as an inexpensive electric source for co-electrolysis initiation was found to contribute to the economy and sustainability of the microreactor for CO2 sequestration and synthesis of organic compounds.
Image: Schematic illustration of the experimental set-up showing microreactor for CO2-sequestration. The microreactor has two inlets perpendicular to each other, one for seawater connected to the syringe pump operating at a constant flow-rate (Qw = 3 mL/min) and another for injection of gaseous CO2 from a pure CO2 gas cylinder with a mass flow meter operating at a constant flow-rate (Qg = 3 mL/min). The integrated Cu electrodes are positioned perpendicular to the gas–liquid flow and connected to a solar panel circuitry. The microreactor operates under sunlight and the organic products are collected at downstream of microchannel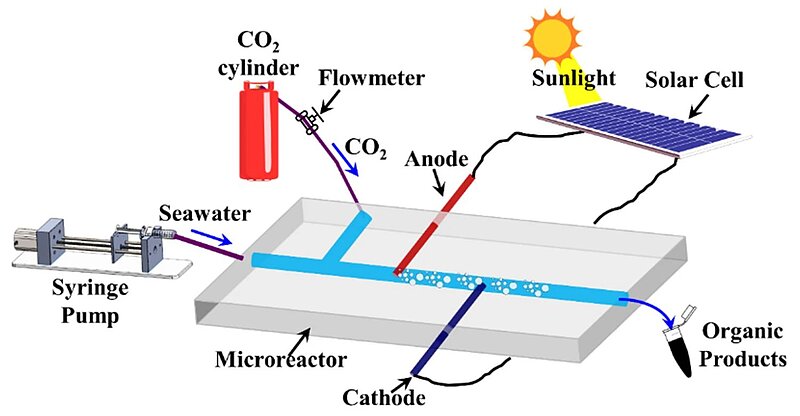
Source: Saptak Rarotra, Saptak Rarotra, Tapas Kumar Mandal & Dipankar Bandyopadhyay/ Co-electrolysis of seawater and carbon dioxide inside a microfluidic reactor to synthesize speciality organics/ Scientific Reports volume 13, Article number: 10298 (2023), 26 June 2023/ doi.org/10.1038/s41598-023-34456-6/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
The reactor device has several advantages: Parallel testing enabled by the new electrochemical flow cells might accelerate the screening of catalysts by up to four times compared to classic approaches. Owing to its modular design, the cells can also be used for other processes. Therefore, the current cell design enables building a variety of reactor configurations, for example, with different electrode spacings, which illustrates the broad range of possible applications. Moreover, other tasks can also be considered in addition to water electrolysis, such as the production of active pharmaceutical ingredients or the decomposition of waste in wastewater treatment. The reactor can be used for determining the best way to opitmise the process of water electrolysis. Another useful application can be determining which electrocatalysts and membranes increase the efficiency of the process.
In general, specific adaptations can be made to the scalable electrochemical microreactors so that they can be used in different types of tasks. Possibly for this reason electrochemistry is currently experiencing a big boost caused by the search for green synthesis processes and efforts towards the direct use of (excessive) sustainably generated energy.
By the Editorial Board
