Aenert. Research Laboratory news
Aqueous zinc-organic batteries (ZOBs) are a fairly modern class of batteries featuring all the advantageous traits of zinc-ion batteries and organic cathode materials: They draw on renewable resources and have a positive impact on CO2 emissions. Especially the tunability of their molecular structure and the diversity of organic reactions which they can produce makes organic materials have infinite potential in the field of energy storage. Organic electrodes are flexible, green and have exhibited fine electrochemical performance. The metal component zinc also adds many benefits to the battery components as it is low cost, safe and has small ionic size.
However, zinc-organic batteries are still struggling with certain issues which have to be overcome before they can be used on a large scale. One of these issues is that they suffer from limited electron conductivity, low energy density, and low cyclability caused by their intrinsic molecular structure, random stacking properties, and inevitable dissolution of functional groups. Therefore, there is great interest in exploring new types of organic cathodes that can overcome the above barriers.
Now (2023), scientists have designed a very efficient zinc-organic battery whose outstanding performance is achieved by controlling the electron delocalisation within a fully conjugated two-dimensional hydrogen-bonded organic framework as a cathode material. The reason for this is that the intermolecular hydrogen bonds turn this framework into a transverse two-dimensional extended stacking network and give it structural stability. Meanwhile, various C = O and C = N electroactive centres interact to trigger multielectron redox chemistry with super delocalisation and thus boost the redox potential, intrinsic electronic conductivity and redox kinetics. It was also found that the fully conjugated molecular configuration brought along reversible Zn2+/H+ synergistic storage together with 10-electron transfer. Thanks to the synergistic effects, the organic cathode delivered a reversible capacity of 498.6 mAh g−1 at 0.2 Ag−1, good cyclability and a high energy density (355 Wh kg−1).
The scientists were able to construct a super-electron-delocalized fully conjugated two-dimensional (2D) hydrogen-bonded organic framework (F-HOF) through applying a nucleophilic functionalization strategy, i.e., the benzo[a]benzo[7,8]quinoxalino[2,3-i]phenazine-5,6,8,14,15,17-hexane (BBQPH) F-HOF featuring synergistic C = O and C = N electron-withdrawing motifs. These motifs were able to change the intramolecular electron distribution and thus boost the redox voltage and trigger multielectron storage chemistry for ZOBs. The multiple intermolecular hydrogen bonds (C = N···H/C = O···H) combined with the π–π stacking interactions (noncovalent orbital overlap between the pi bonds of aromatic rings) could also improve the structural stability of BBQPH. Moreover, the super electron delocalisation also enhanced the redox potential, intrinsic electronic conductivity, and redox kinetics. The BBQPH electrodes were also found to deliver a high voltage of 1.2 V, a improved capacity (498.6 mAh g−1 at 0.2 A g−1), and good cycling performance of >1000 cycles at 5.0 A g−1 for aqueous ZOBs. Also, ex situ investigations and theoretical simulations were conducted to gain deeper insights into the reversible Zn2+/H+ synergistic storage mechanism accompanied by 10-electron transfer.
In addition, to precisely make out the electron delocalisation of the designed molecules, the electrostatic potential (ESP) and molecular orbitals were first calculated to evaluate the intrinsic electronic properties. Compared to BBQPD, the ESP value (−0.058 a.u.) of o‑quinone functional groups (derived from aromatic compounds by conversion of an even number of –CH= groups into –C(=O)– groups, resulting in a fully conjugated cyclic dione structure) for BBQPH was decreased because of its super electron delocalisation and highly conjugated symmetric structure. Meanwhile, BBQPH exhibited greater dramatic electron delocalisation, which resulted in smaller molecular dipole moments and robust intramolecular electron migration from the conjugate planar center to the o‑quinone groups. There was found to exist an increased density of electron states at the Fermi level for the designed BBQPH according to the energy band and partial density of states, which was assumed to be primarily contributed by C=O group. This proved that o‑quinone increased the super electron delocalisation and also underlined the potential for fast charge transfer of BBQPH. Therefore, the elaborately tailored BBQPH was found to be significantly attractive for ZOBs.
Image: a Scheme of the composition of the electrode potentials (µA and µC), which are related to the electron energy of molecular orbitals. The open-circuit voltage for ZOBs depends on the HOMO energy of the organic cathode: Voc = µA−µC. b Chemical structures of BBQPD and BBQPH. c Simulated ESP distributions of BBQPD and BBQPH. d Calculated relative HOMO/LUMO energy levels and energy gaps used in the DFT method. e Energy band spectrum and corresponding pDOS of the simulated BBQPH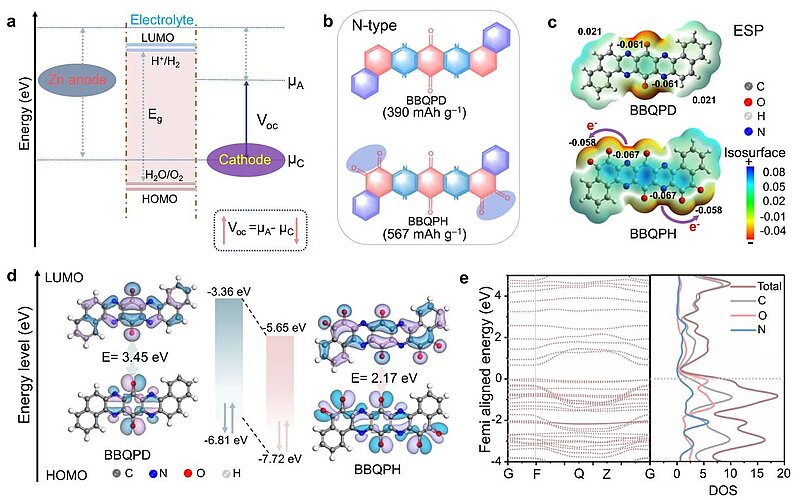
Source: Wenda Li, Hengyue Xu, Hongyi Zhang, Facai Wei, Lingyan Huang, Shanzhe Ke, Jianwei Fu, Chengbin Jing, Jiangong Cheng & Shaohua Liu/ Tuning electron delocalization of hydrogen-bonded organic framework cathode for high-performance zinc-organic batteries/ Nature Communications volume 14, Article number: 5235 (2023), 28 August 2023/ doi.org/10.1038/s41467-023-40969-5/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
Scientists are avidly working on advancing the organic zinc battery technology. In 2023, sandwich-structured perylene diimide-ethylene diamine/graphene (PDI-EDA/EG) composites were analysed for their suitability for battery applications. The two-dimensional graphene host was found to be able to interact with the PDI-EDA polymer through π–π stacking, thus enabling accelerated ion/electron transfer, many active sites, structural integrity, and mitigated solubility of the hybrid electrodes. The hybrid electrode exhibited a high capacity (superior rate capability, and high durability (93.4% capacity retained after 1000 cycles). The structure evolution of the hybrid electrode during the insertion/extraction cycle was analysed by ex-situ Fourier transform infrared spectra (FTIR) and X-ray photoelectron spectroscopy (XPS). It revealed a reversible Zn2+ storage at carbonyl sites. Moreover, a prototype rocking-chair ZIB cell was constructed having a zinc pre-intercalated MnO2 cathode, which displayed an ultrahigh energy density of 54.9 Wh kg−1 at a power density of 42.5 W kg−1 and high stability with little capacity decay after 1000 cycles.
Image: (a) Schematic of PDI-EDA/EG composite preparation. (b) XRD patterns, (c) FTIR spectra, and (d) Raman spectra of EG, PDI-EDA, PDI-EDA/EG-20. (e–h) SEM images of PDI-EDA, PDI-EDA/EG-10, PDI-EDA/EG-20, and PDI-EDA/EG-30 samples, respectively
Source: Yuyan Tang, Shaohui Li, Meng-Fang Lin, Jingwei Chen/ A π–π Stacked High-Performance Organic Anode for Durable Rocking-Chair Zinc-Ion Battery/ Batteries 9(6):318, June 2023/ DOI:10.3390/batteries9060318/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2023, a nanocellulose‐carboxymethylcellulose (CMC) hydrogel electrolyte was created which exhibited stable cycling performance and high Zn²⁺ conductivity (26 mS cm⁻¹). This was attributed to the material's strong mechanical strength and water‐bonding ability. Using this electrolyte, the Zn‐metal anode had high cycling stability at a high rate, with the ability to maintain a current density as high as 80 mA cm⁻² for more than 3500 cycles and a cumulative capacity of 17.6 Ah cm⁻² (40 mA cm⁻²). Moreover, side reactions, including hydrogen evolution and surface passivation, were reduced because of the strong water‐bonding capacity of the CMC. Full Zn||MnO2 batteries incorporating this electrolyte demonstrated very good high‐rate performance and long‐term cycling stability.
Image: Cellulose‐CMC electrolyte for aqueous Zn ion batteries. A) When the electrolyte membrane is made of pure cellulose, there is either too much free water that causes parasitic side reactions, or too little water after drying that causes low Zn²⁺ conductivity. B) In contrast, by adding CMC within the cellulose matrix, after a squeeze‐dry process to reduce the amount of free water molecules and to mitigate parasitic side reactions, there are still water molecules bonded along the CMC chains, which enables the transport of the Zn ions
Source: Lin Xu, Taotao Meng, Xueying Zheng, Tangyuan Li/ Nanocellulose‐Carboxymethylcellulose Electrolyte for Stable, High‐Rate Zinc‐Ion Batteries/ Advanced Functional Materials 33(27), April 2023/ DOI:10.1002/adfm.202302098/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
Zinc-organic batteries have several advantages: Through the super electron delocalisation, the intramolecular electron distribution is altered, which, in turn, significantly increases the redox potential. A high output voltage in combination with a high capacity is responsible for a high energy density of batteries. Organic electrode molecules as cathode materials are particularly suitable for batteries due to their environmental friendliness, sustainability, structural designability and abundance.
Aqueous zinc-organic batteries are promising and fairly new technology for battery design. They are beneficial for the environment and draw on abundant resources. However, it may still take several years before a fully-deployable battery is ready for market launch as problems pertaining to their stable performance have yet to be solved.
By the Editorial Board
