In search of an easily rechargeable energy storage medium, many types of batteries have been developed in the past decades. The most common among them undoubtedly is the well-known and widely-used lithium-ion battery. However, in view of the uneven geographic distribution, negative environmental impact and high cost of many of the elements required for lithium-ion batteries, scientists have increasingly been looking for alternatives. One such alternative is the sodium-ion battery. Its working principle and cell construction are very similar to those of commercially-available lithium-ion batteries. Moreover, sodium is a very abundant material compared to lithium, which would contribute to reducing the production costs of batteries. On the downside, there still remain several challenges which hamper the widespread application of sodium-ion batteries, including, for example, low energy density and a very limited number of charge-discharge cycles.
Now (2022), scientists at Argonne National Laboratory may have discovered one of the main reasons for the decreased performance in sodium-ion batteries. They found that this decrease was likely caused by the defects forming during preparation of the cathode material. The defects were responsible for structural earthquakes in the cathode, which resulted in a dramatic performance decline in the course of battery cycling. The discovery was made using the resources available at Argonne’s Center for Nanoscale Materials (CNM) and Advanced Photon Source (APS).
In order to synthesise the cathode, the scientists slowly heated the cathode material to a high temperature in air, kept it at this temperature for a certain time and then dropped it again to room temperature. The data they received was that a rapid temperature drop during material synthesis made the cathode particle surface less smooth and induced strain in large areas of the material. The data also showed that a push-pull effect in these areas took place during cathode cycling, causing a cracking of the cathode particles and a performance decline. Upon further study, the team found that this degradation became stronger when the cathodes were cycled at higher temperature (54.4 C°) or during fast charging (one hour instead of 10 hours).
Image: Solid-state synthesis of O3 NaNi0.4Mn0.4Co0.2O2: 2D contour plot of in situ SXRD patterns during a heating, b holding, and c quenching process. The color in a–c represents the intensity, with red for highest and blue for lowest. d Lattice parameter a, e lattice parameter c and f microstrain evolution during the heating/holding/quenching process. g SXRD Rietveld refinement, h SEM image and i cross-section SEM image of the strained O3 NaNi0.4Mn0.4Co0.2O2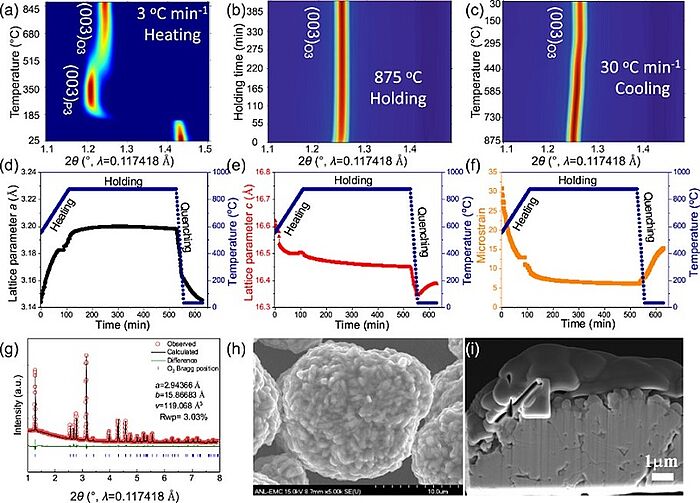
Source: Yangbin Shen, Yulu Zhan, Shuping Li, Fandi Ning, Ying Du, Yunjie Huang, Ting He and Xiaochun Zhou/ Native lattice strain induced structural earthquake in sodium layered oxide cathodes/ Nature Communications volume 13, Article number: 436 (2022), 27 January 2022/ doi.org/10.1038/s41467-022-28052-x/ Open Access This article is licensed under a Attribution 4.0 International (CC BY 4.0)
For many years, scientists have been working relentlessly on finding cheaper and readily available alternatives to lithium-ion batteries. In 2019, scientists found that zinc-doped manganese based layered oxides (Na0.833[Li0.25Mn0.75]O2) can not only suppress the Jahn–Teller effect, which leads to a geometric distortion of a non-linear molecular system that reduces its symmetry and energy, but also reduce the inherent phase separations. They also discovered that the zinc-doped sample had lower formation energy, more stable ground states, and fewer spinodal decomposition regions than those of the undoped sample which enabled charging or discharging without any phase transition. The zinc-doped sample exhibited superior cycling performance and demonstrated that zinc doping was an effective strategy for developing high-performance layered cathode materials.
Image: Manganese based layered oxides with modulated electronic and thermodynamic properties for sodium ion batteries: Theoretical calculations regarding the effects of Zn doping in the fully sodiated Na[Li0.25Mn0.75]O2. a Shows the Mn3+ sites as purple octahedra in the relaxed crystal structure of Na[Li0.25Mn0.75]O2 together with its corresponding PDOS. Likewise, the Mn4+ sites are shown as purple octahedra in b together with its corresponding PDOS. c Shows PDOS of the Mn3+ sites in the Zn-doped Na[Li0.25Mn0.75]O2. Likewise, PDOSs of the Zn2+ and the Mn4+ sites in the Zn-doped Na[Li0.25Mn0.75]O2 are shown in d, e, respectively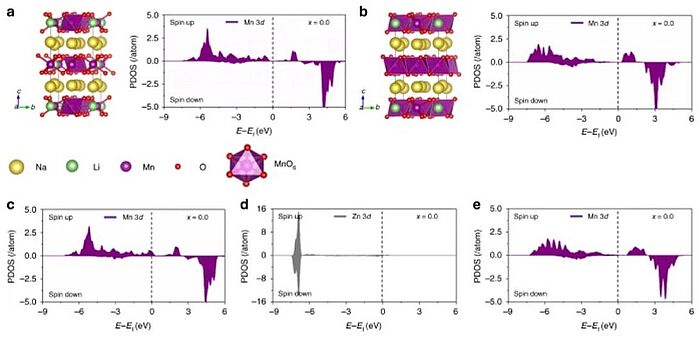
Source: Kai Zhang, Duho Kim, Zhe Hu, Mihui Park, Gahee Noh, Yujeong Yang, Jing Zhang, Vincent Wing-hei Lau, Shu-Lei Chou, Maenghyo Cho, Si-Young Choi & Yong-Mook Kang/ Manganese based layered oxides with modulated electronic and thermodynamic properties for sodium ion batteries/ Nature Communications volume 10, Article number: 5203 (2019), 07 January 2019/ doi.org/10.1038/s41467-018-07646-4/ Open Access This article is licensed under a Attribution 4.0 International (CC BY 4.0)
In 2021, scientists wanted to find a solution to the limited capacity and inferior phase transition of sodium-ion batteries due to the gliding of transition-metal layers upon extraction and insertion of positive natrium ions in the cathode materials and found that the large-sized positively-charged potassium ion was riveted in the prismatic positive sodium ion sites of P2-Na0.612K0.056MnO2 to enable more thermodynamically favourable positive sodium ion vacancies. The manganese and oxygen bonds were strengthened to reduce phase transition during charge and discharge. 0.901 positive sodium-ions per formula were alternately extracted and inserted, in which only the two-phase transition of P2 ↔ P’2 took place at low voltages. The battery showed the highest specific capacity of 240.5 mAh g−1 and energy density of 654 Wh kg−1 and a capacity retention of 98.2% after 100 cycles. This research provided valuable insights into the tuneable chemical environments of transition-metal oxides for advanced cathode materials and promoted the development of sodium-ion batteries.
Image: Tuning local chemistry of P2 layered-oxide cathode for high energy and long cycles of sodium-ion battery: a XRD pattern and Rietveld refinement. Typical layered structure of Na0.612K0.056MnO2 viewed along the y axis (b) and the z axis (c) with K+ located at the Nae sites. d PDF pattern and structure of Na0.612K0.056MnO2. The representative peaks correspond to the bond length of Mn–O (blue line) and Mn–Mn (pale blue line) as labeled. e Fitting of Mn K-edge FT-EXAFS spectra of Na0.612K0.056MnO2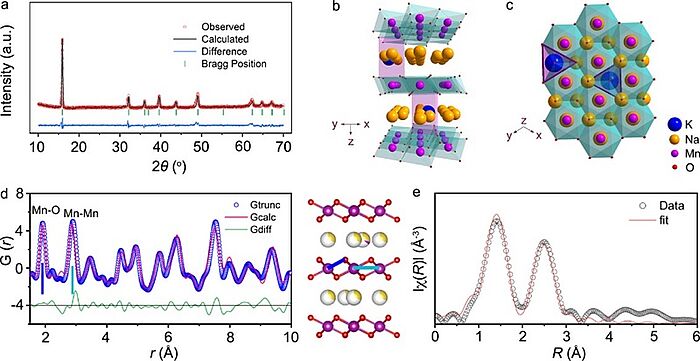
Source: Chenchen Wang, Luojia Liu, Shuo Zhao, Yanchen Liu, Yubo Yang, Haijun Yu, Suwon Lee, Gi-Hyeok Lee, Yong-Mook Kang, Rong Liu, Fujun Li & Jun Chen/ Tuning local chemistry of P2 layered-oxide cathode for high energy and long cycles of sodium-ion battery/ Nature Communications volume 12, Article number: 2256 (2021), 15 April 2021/ doi.org/10.1038/s41467-021-22523-3/ Open Access This article is licensed under a Attribution 4.0 International (CC BY 4.0)
Improving the performance of sodium-ion batteries has several potential benefits: sodium is an attractive material because of its greater abundance and lower cost compared with lithium. Moreover, a sodium-ion battery can greatly increase the amount of energy that can be stored in a given weight or volume when it is cycled at a high voltage of about 4.5 volts. However, its fairly rapid performance decline with charge-discharge cycling has so far hindered widespread commercialisation.
Earlier research carried out by the same team of researchers has already yielded a greatly improved battery anode. The scientists are confident that when they match the improved cathode with the already improved anode they will get a 20% - 40% increase in performance. This would eventually result in a longer driving range and more affordable electric vehicles, but also lower the costs for energy storage on the electric grid.