Aenert. Research Laboratory news
It is common knowledge that cold weather and electric batteries do not go together well as most of them lose their efficiency in cool temperatures. This is mainly due to the liquid electrolyte contained in lithium-ion batteries. This battery component transfers ions between two electrodes which causes the battery to charge and discharge. At temperatures below zero, however, the liquid begins to freeze thus negatively impacting the charging ability of electric vehicles.
Now (2023), a new composition may have successfully tackles this problem. Scientists at the Argonne and Lawrence Berkeley national laboratories have developed a fluorine-containing electrolyte that performs well even in sub-zero temperatures. This quality might be used for electric vehicles, as well as in energy storage for electric grids and consumer electronics like computers and phones.
In lithium-ion batteries, the electrolyte consists of a widely available salt (lithium hexafluorophosphate) and carbonate solvents such as ethylene carbonate. During charging, ions move out of the cathode, pass through the electrolyte and finally arrive at the anode. While travelling through the electrolyte, they sit at the center of clusters of four or five solvent molecules. When these clusters strike the anode surface for the first time, they form a protective layer, a so-called solid-electrolyte interphase, which, like a filter, lets only the lithium ions pass through the layer while blocking the solvent molecules. In cold temperatures, however, the electrolyte with carbonate solvents begins to freeze. It loses the ability to transport lithium ions into the anode on charge. The ions now need much higher energy to evacuate their clusters and penetrate the interface layer than at room temperature.
This is why the scientists wanted to find a new, more temperature-resilient electrolyte to evade this problem. Therefore, they investigated several fluorine-containing solvents and found a composition which had a low energy barrier for releasing lithium ions from the clusters at sub-zero temperature. They were also able to have a look at why that particular composition worked so well at the atomic scale. It all depended on the position of the fluorine atoms within each solvent molecule and their number.
In testing with laboratory cells, the team’s fluorinated electrolyte was shown to retain stable energy storage capacity for 400 charge-discharge cycles at minus 20°C. More so, even at that sub-zero temperature, the capacity was equivalent to that of a cell with a conventional carbonate-based electrolyte at room temperature.
Image: a) Scheme of solvent design transition from carbonates to fluorinated esters. b) Atomic charge analysis of carbonyl groups in EA, EA-f, f-EA, and f-EA-f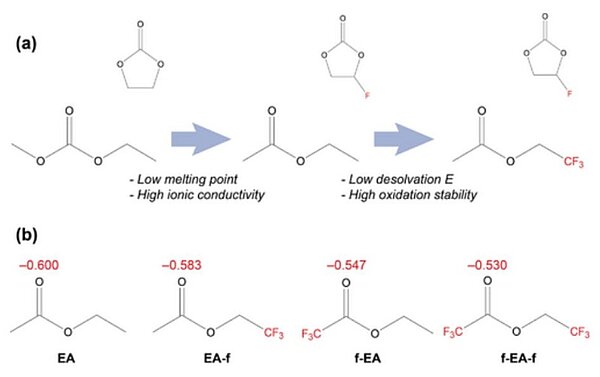
Source: Dong-Joo Yoo, Qian Liu, Orion Cohen, Minkyu Kim, Kristin A. Persson, Zhengcheng Zhang/ Rational Design of Fluorinated Electrolytes for Low Temperature Lithium-Ion Batteries/ Advanced Energy Materials, Volume 13, Issue 20, May 25, 2023/ doi.org/10.1002/aenm.202204182/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International (CC BY-NC 4.0)
Making batteries more resistant to the cold and other extreme weather conditions is one of the chief interests of modern car battery design. In 2022, scientists analysed three activation methods of the nickel cathode in a molten-salt battery, including: a. heat treating the cathode granules under H2/N2, b. incorporating a partially charged NiCl2/Ni cathode, and c. doping the molten salt electrolyte with sulphur. Particularly sulphur doping, a cost-efficient method suitable for large-scale applications, was found to be not only effective in activating the Ni cathode initially but also suitable for energy retention during thermal cycling. In general, Al-Ni molten salt batteries under thermal cycling were shown to have high retention in cell capacity over weeks.
Image: Design and mechanism of an Al-Ni battery: Thermal-cycling cell design for Al-Ni battery where the cathode (Ni) and anode (Al) are separated with a glass microfiber disk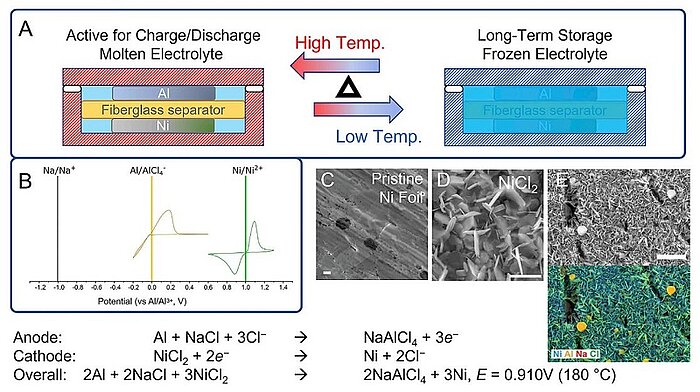
Source: Minyuan M Li, Xiaowen Zhan, Evgueni Polikarpov, David M Reed/ A freeze-thaw molten salt battery for seasonal storage/ Cell Reports Physical Science 3(4):100821, May 2022/ DOI:10.1016/j.xcrp.2022.100821/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2023, scientists designed aramid nanofiber (ANF)‐reinforced poly(vinyl alcohol) (PVA) organohydrogels containing dimethyl sulfoxide (DMSO)/H2O mixed solvents with outstanding freeze‐resistance by means of solution casting and 3D printing methods. The organohydrogels exhibited high tensile strength as well as toughness due to the synergistic effect of ANFs and DMSO in the system, which was responsible for PVA crystallization and intermolecular hydrogen bonding interactions between PVA molecules as well as ANFs and PVA. The organohydrogels also showed ultrahigh ionic conductivity, ranging from 1.1 to 34.3 S m−1 at −50 to 60°C. On this basis, the organohydrogel‐based strain sensors and solid‐state zinc–air batteries (ZABs) were fabricated, which have a broad working temperature range. Especially, the ZABs did not only have high specific capacity (262 mAh g−1) with ultra‐long cycling life (355 cycles, 118 h) even at −30°C, but were also shown to work properly under various deformation states. Organohydrogels simultaneously with high mechanical properties, ionic conductivity, and outstanding freeze resistance were then used to fabricate flexible strain sensors and solid‐state zinc–air batteries (ZABs) with excellent comprehensive properties. The organohydrogel‐based ZABs exhibited high energy capacity and ultra‐long cycling life both at room temperature and −30°C.
Image: Schematic illustration of a) the preparation procedures of the ANF‐PVA organohydrogels by solution casting in a mold and 3D printing; b) the formation and microstructure of ANF‐PVA organohydrogels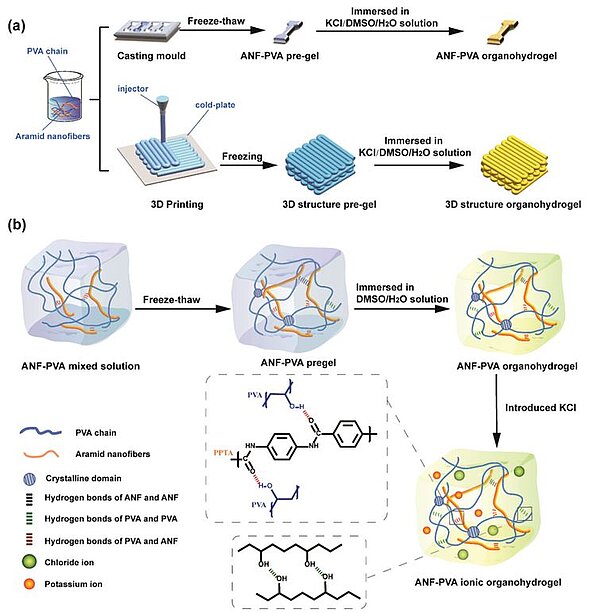
Source: Jiayu Lyu, Qingya Zhou, Haifeng Wang, Qi Xiao/ Mechanically Strong, Freeze‐Resistant, and Ionically Conductive Organohydrogels for Flexible Strain Sensors and Batteries/ Advanced Science 10(9):2206591, January 2023/ DOI:10.1002/advs.202206591/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
The antifreeze electrolyte has many advantageous properties. Apart from working efficiently in low temperatures, it is also safer than the carbonate-based electrolytes currently used as it does not catch fire. The research was able to demonstrate how the atomic structure of electrolyte solvents could be modified to design new electrolytes for sub-zero temperatures.
Currently, the team of researchers is patenting the low-temperature and safer electrolyte and is also searching for an industrial partner to adapt it to one of their designs for lithium-ion batteries and eventually launch it onto the market.
By the Editorial Board