Aenert. Research Laboratory news
Alongside increased efforts to come up with new, more abundant and also more sustainable materials to create batteries, scientists are also trying to improve existing battery types by enhancing their stability and durability. This led them to have a closer look at an ingredient found in many toothpastes. The ingredient is called sodium fluoride and is a compound of fluorine. Sodium fluoride is an inorganic compound having the formula NaF. Colorless or white, it is readily soluble in water and used in trace amounts in the fluoridation of drinking water to prevent tooth decay.
The scientists at the U.S. Department of Energy’s (DOE) Argonne National Laboratory now took this toothpaste ingredient (2023) and transferred the compound to lithium-metal car batteries where it was found to help against performance decline. As non-lithium-ion batteries offer twice or more energy stored in a given volume or weight compared to lithium ion, cars could be powered for much longer distances and could even power long-haul trucks and aircraft one day. The use of such batteries would also help mitigate climate change.
The battery had an anode consisting of lithium metal instead of graphite which is normally used. The cathode consisted of nickel, manganese and cobalt (NMC). To improve battery efficiency, the team of scientists changed the electrolyte and thus were able to overcome the problem of short life cycles commonly witnessed in lithium-metal batteries. In this type of battery, the electrolyte usually does not form an adequate protective layer on the anode surface during the first few cycles which eventually leads to declining battery efficiency. The so-called solid-electrolyte-interphase is supposed to act like a guardian while the lithium ions pass in and out of the anode to charge and discharge the battery, respectively.
The team found a new fluoride solvent which was proved to maintain a robust protective layer for hundreds of cycles. It combined a fluorinated component with a positive charge with a different fluorinated component having a negative charge. The main difference in the new electrolyte was the substitution of fluorine for hydrogen atoms in the ring-like structure of the cation part of the ionic liquid. In order to gain greater insight into the mechanism lying at the bottom of this difference at the atomic scale, the team relied on the high-performance computing.
Simulations on the ALCF’s Theta supercomputer revealed that the fluorine cations accumulated on the anode and cathode surfaces before any charge-discharge cycling. Then, during the early stages of cycling, a resilient SEI layer was built up on the surface which was found to surpass the possibilities with previous electrolytes. High-resolution electron microscopy at Argonne and Pacific Northwest National Laboratory showed that the highly protective SEI layer on the anode and cathode led to the stable cycling.
Also, the team was able to find the perfect proportion of fluoride solvent to lithium salt to create a layer with optimal properties. Because of this layer, lithium ions could efficiently flow in and out of the electrodes during charge and discharge for hundreds of cycles.
Image: SEM of plated Li on Cu foil with current density of 0.1 mA/cm2
Source: Qian Liu, Wei Jiang, Jiayi Xu, Yaobin Xu, Zhenzhen Yang, Dong-Joo Yoo, Krzysztof Z. Pupek, Chongmin Wang, Cong Liu, Kang Xu/ A fluorinated cation introduces new interphasial chemistries to enable high-voltage lithium metal batteries/ Nature Communications volume 14, Article number: 3678 (2023), 21 June 2023/ doi.org/10.1038/s41467-023-38229-7/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
Scientists have long tried to improve lithium-ion batteries. In 2016, a lithium ion battery using an aqueous electrolyte solution was analysed. It was made using graphite coated with gel polymer membrane and LISICON as the negative electrode, and LiFePO4 in aqueous solution as the positive electrode. Its average discharge voltage amounted up to 3.1 V and energy density based on the two electrode materials was 258 Wh kg−1. During the same charge-discharge process, the temperature of the system was found to be more stable in comparison with that of the conventional lithium-ion batteries. The aqueous electrolyte was placed in direct contact with both the negative and the positive electrodes. Therefore, the cooling effects were very efficient and a cooling system, usually needed for large capacity battery modules, was not needed for the application of this battery in electric vehicles. The scientists also found that if other intercalation compounds such as LiMn2O4, LiCoO2 and Li[Ni1/3Co1/3Mn1/3] O2, stable in aqueous electrolytes, were used as the positive electrode, the average discharge voltage was found to be higher as well as the energy density and cycling performance improved. The composite polymer membrane was flame retarding and became a gel after saturating with the organic electrolyte. All in all, the battery system was found to be a promising energy storage system.
Image: Schematic illustration of our designed ALIB using the graphite coated by GPE and LISICON as negative electrode, LiFePO4 in 0.5 mol l−1 Li2SO4 aqueous electrolyte as positive electrod
Source: Zheng Chang, Li Chunyang, Yanfang Wang, Bingwei Chen/ A lithium ion battery using an aqueous electrolyte solution/ Scientific Reports 6(1):28421, June 2016/ DOI:10.1038/srep28421/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2021, scientists introduced a novel method for obtaining nanostructures for LIB anode application on various surfaces via nanotransfer printing (nTP) process. A spark plasma sintering (SPS) process was employed to fabricate a sputter target made of Li2CO3, which was used as an anode material for the LIBs. Using the nTP process, various Li2CO3 nanoscale patterns, such as line, wave, and dot patterns on a SiO2/Si substrate, were successfully obtained. Furthermore, the scientists tested highly ordered Li2CO3 nanostructures on a variety of substrates, such as Al, Al2O3, flexible PET, and 2-Hydroxylethyl Methacrylate (HEMA) contact lens substrates. They assumed that their approach would provide new pathways to generate many other designable structures of various LIB anode materials.
Image: Fabrication of a Li2CO3 sputter target using a Spark Plasma Sintering (SPS) system. (a) Schematic image of the SPS system. (b) Photographic image of the working SPS system for the fabrication of the Li2CO3 sputter target. (c) Li2CO3 raw material (powder). (d) Three-inch Li2CO3 sputter target fabricated by the SPS process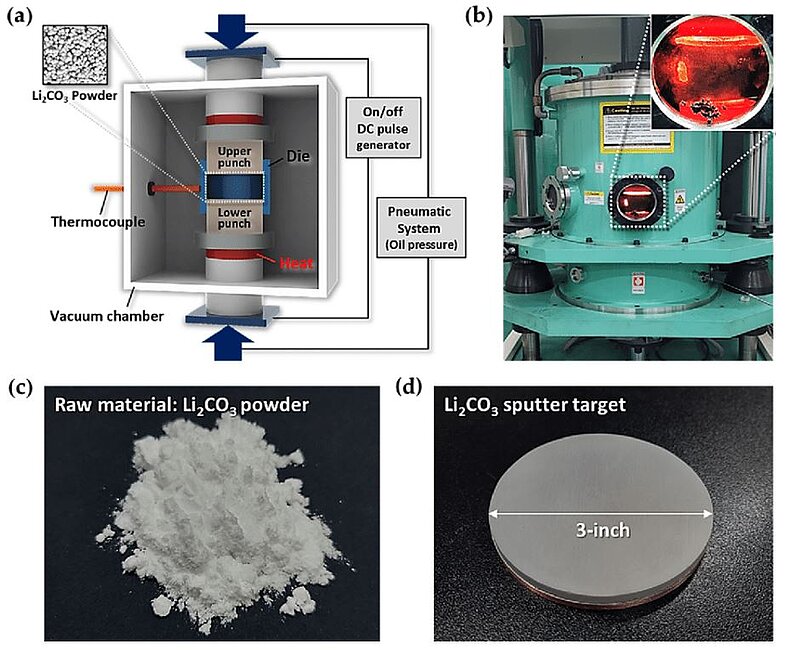
Source: Tae Wan Park, Young Lim Kang, Sang Hyeon Lee, Gu Won No/ Formation of Li2CO3 Nanostructures for Lithium-Ion Battery Anode Application by Nanotransfer Printing/ Materials 14(7):1585, March 2021/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
There are many advantages involved in employing the new electrolyte: It is relatively cheap in its manufacturing as it can be made with very high purity and yield in one step rather than multiple steps. Also, it is environmentally friendly because it uses much less solvent, which is volatile and can release contaminants into the environment. It is also safer because it is not flammable.
The scientists are convinced that lithium metal batteries with the addition of the new fluorinated cation electrolyte could considerably boost the electric vehicle industry. Moreover, the usefulness of this electrolyte was proved to extend to other types of advanced battery systems beyond lithium ion.
By the Editorial Board
