埃纳特。研究实验室新闻
在当今的工业化世界中,氢在农业和工业等许多领域发挥着重要作用。氢气具有减少二氧化碳排放的潜力,因为其生产和燃烧都不会将CO2排放到大气中。而且,它还可以转化为电力或合成气体。然而,迄今为止,它仍然主要由化石燃料产生。化石燃料是CO 2排放的罪魁祸首之一,因此会加剧气候变化问题。因此,科学家们正在不懈地寻找利用氢的力量而不产生有害物质的方法。
现在( 2023年),特拉维夫大学的科学家在可持续氢气的生产方面取得了重大突破,因为他们的方法不仅避免了空气污染,而且被证明是高效的。
在植物中,通常是太阳为植物酶提供动力,将水分子分解成气体。这称为氢化酶。以色列科学家现在开发出一种可以轻松地用电力替代太阳能的方法。唯一的问题是他们必须找到解决酶自然被电荷排斥的问题的方法。因此,科学家团队创造了一种可以阻止这种反应的化学处理方法:与氢化酶混合的水凝胶。
水凝胶用于将酶附着在电极上,因此可以在生物催化剂的帮助下产生氢气,效率超过 90%,这意味着引入系统的 90% 的电子沉积在氢气中,无需任何二次过程。
这项研究的伟大成就是他们采用了一种已知的物质——水凝胶,并将其重新用于生产氢气。他们将电极浸泡在凝胶中,其中含有产生氢气的酶。研究发现,即使在电压下,凝胶也能够长时间保留酶,从而能够不受抑制地产生氢气。当将凝胶放入水中时,凝胶形成纳米纤维,能够将酶粘附到电极上,从而防止酶被电荷排斥。除了氢化酶之外,还用其他两种酶对凝胶进行了测试,并证明它能够将不同的酶附着到电极上。
Image: Electrochemical properties of HydA encapsulated in an FmocFF-soaked electrode. (A) Proposed scheme of the electrochemical activity at different potentials. Yellow triangles represent MVox, blue triangles represent MVred, HydA is represented by its protein crystallographic structure, light blue circles represent H2, and the dark-gray and light-yellow areas, respectively, represent the carbon felt electrode soaked with the FmocFF hydrogel. I. Reduction of MV by the electrode and shuttling of electrons to HydA, which catalyzes H2 production. II. Oxidation of the MV pool at the electrode. III. H2 oxidation by HydA reduces MV, which shuttles the electrons back to the electrode. (B) Cyclic voltammogram of the FmocFF hydrogel soaked on a carbon felt electrode supplemented with HydA and MV. (C) Accumulated H2 produced overnight (O.N.) by the electrochemical assay versus the amount of enzyme loaded on the working electrode in the FmocFF hydrogel (red circles), the FmocLL hydrogel (blue tringles), and the solution (black diamonds). Error bars represent mean ± SD of at least six independent experiments. (D–G) Corresponding chronoamperometries of O.N. electrochemical assays of FmocFF-, FmocLL-, and solution-soaked electrodes (red, blue, and black, respectively)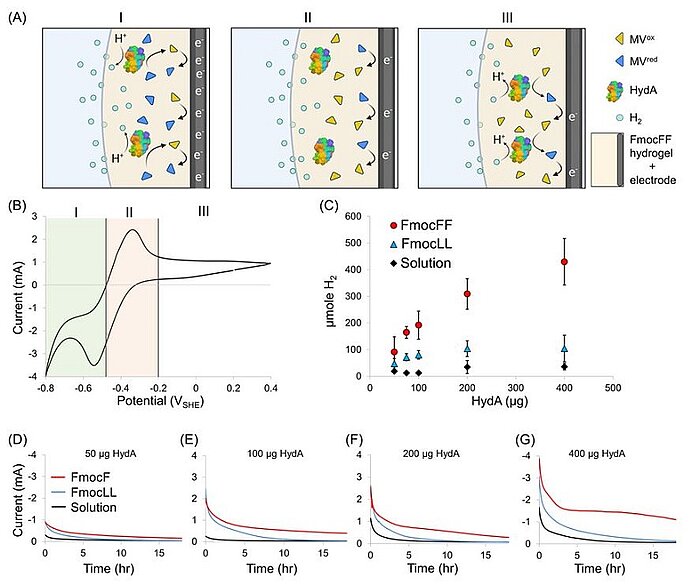
Source: Itzhak Grinberg, Oren Ben-Zvi, Lihi Adler-Abramovich, Iftach Yacoby/ Peptide self-assembly as a strategy for facile immobilization of redox enzymes on carbon electrodes/ Carbon Energy, 11 July 2023/ doi.org/10.1002/cey2.411/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
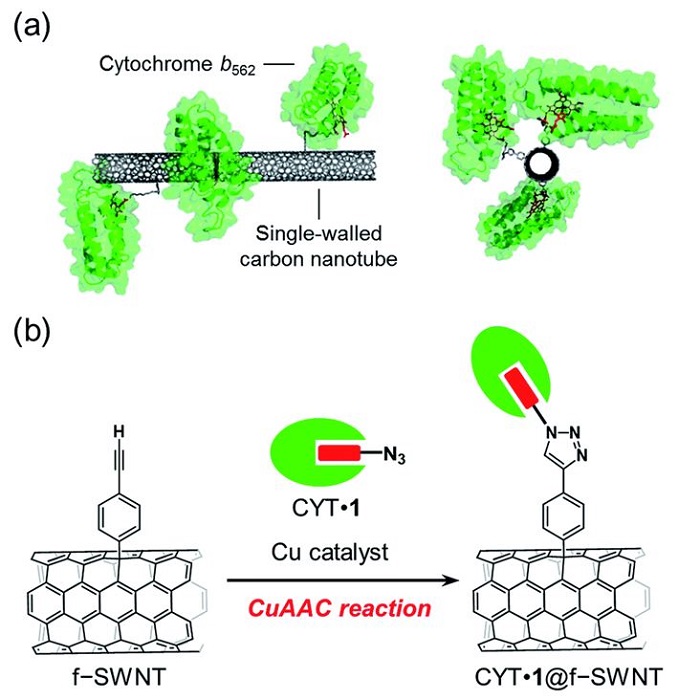
长期以来,科学家们一直在努力寻找高效且廉价地生产氢气的方法。例如, 2016 年,科学家们研究了使用 CuAAC 反应将叠氮化物连接的细胞色素 b562 (CYT) 特异性定向共价固定在单壁纳米管的侧壁上,这是一种点击化学反应,使用高效可靠的反应,例如 Cu(I )-催化的叠氮化物-炔环加成,以结合两个分子结构单元。这种使用连接到叠氮化物部分的可替换血红素的方法的主要优点是野生型血红素蛋白功能化的广泛应用。这种方法使得定向氧化还原活性血红素蛋白单壁纳米管杂化材料的开发成为可能。制造血红素蛋白-碳纳米材料电极的方法被证明在制备专门设计的生物电极界面方面具有巨大的潜力。
Image: (a) SWNT with covalently-linked cytochrome b562 and (b) the preparation scheme using a copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction
Source: Akira Onoda, Nozomu Inoue, Stéphane Campidelli and Takashi Hayashi/ Cofactor-specific covalent anchoring of cytochrome b562 on a single-walled carbon nanotube by click chemistry/ RSC Advances, Issue 70, 2016, 04 Jul 2016 / DOI doi.org/10.1039/C6RA14195A/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
2020年,科学家研究了 [4Fe–4S]H 氧化还原行为的 pH 依赖性。他们使用两种不同的 [FeFe] 氢化酶进行红外 (IR) 光谱电化学分析:来自莱茵衣藻(一种藻类)的 CrHydA1 和来自巴氏梭菌(一种厌氧细菌)的 CpHydA1。他们发现,在我们的实验条件下,[4Fe-4S]H 的氧化还原电位与两种酶生理值附近的 pH 无关。还研究了 CpHydA1 中 [4Fe–4S]H 和附属 [4Fe–4S] 簇(F 簇)之间的氧化还原反协同行为,该行为可以改变活性位点的催化作用。结果表明,在[FeFe]氢化酶的催化循环中,[4Fe-4S]H及其附近没有发生质子化,而是发生了[2Fe]H的质子化催化过程。
Image: Structure and catalytic cycle of [FeFe] hydrogenase. (A) Structure of the H-cluster. (B) Structure of CrHydA1 apoprotein (PDB ID 3LX4) (14) with the H-cluster modeled from the CpHydA1 structure (PDB ID 4XDC). (15) The H-cluster is composed of the [4Fe–4S]H and [2Fe]H subclusters and is shown in a ball-and-stick representation, along with the cysteine ligating [4Fe–4S]H. (C) Structure of CpHydA1 artificially maturated with the diiron ADT cofactor (PDB ID 4XDC). (15) The H-cluster and accessory F-clusters are shown as balls and sticks, and the protein backbone is shown as a cartoon. The H-domain harboring the H-cluster in both enzymes is colored blue, and the F-domain containing the accessory F-clusters, only present in CpHydA1, is colored pink. (D) Simple form of Model 1 proposed for the catalytic cycle, where [4Fe–4S]H is represented by a diamond and [2Fe]H is represented as a rectangle. The CN– and terminal CO ligands are omitted for clarity. The H-cluster containing the propane 1,3-dithiolate (PDT) ligand with methylene in the bridgehead only cycles between Hox and Hred, while the H-cluster containing ADT participates in the full cycle. A recent work of Lorent and coworkers has uncovered additional forms of the Hhyd state. (16) (E) Model 2 of the catalytic cycle (2) where the Hox state becomes reduced and protonated on [4Fe–4S]H simultaneously to give Hred’. Hred’ is then reduced and protonated simultaneously to give Hhyd, where the proton on [4Fe–4S]H is retained. Hhyd then acquires an additional proton, reacting to make H2, leaving a proton on [4Fe–4S]H in the HoxH state. The Hred and Hsred states are inactive, bridging hydride containing states. Hred’ can also be protonated at an alternative site near [4Fe–4S]H to give the Hred’H state. Hred’H is also known to be the reduced and protonated form of HoxH. In this model, the H-cluster containing the ADT ligand can participate in the full cycle, while the PDT containing H-cluster can only access the Hox, Hred’, HoxH, and Hred’H states. Cycle 2 was adapted from three related models appearing in previous works, while trying to fit Hred’H into its most logical location. (2,3,17)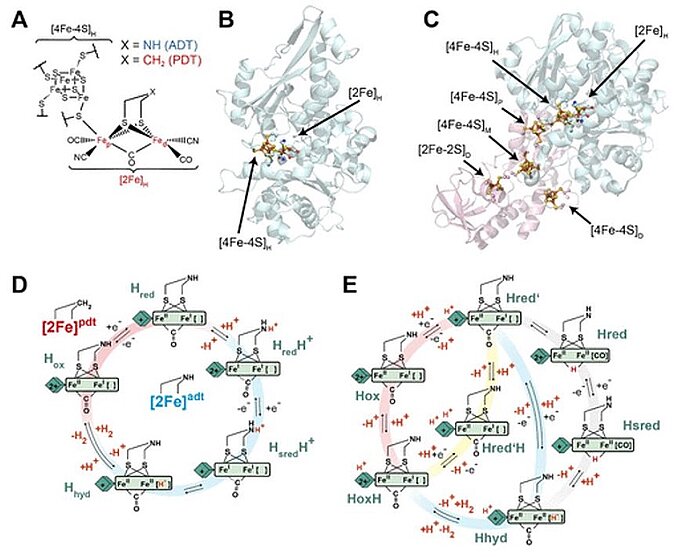
Source: Patricia Rodríguez-Maciá, Nina Breuer, Serena DeBeer, James A. Birrell/ Insight into the Redox Behavior of the [4Fe–4S] Subcluster in [FeFe] Hydrogenases/ ACS Catal. 2020, 10, 21, 13084–13095, October 27, 2020/ doi.org/10.1021/acscatal.0c02771/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
使用水凝胶生产氢气有几个好处:即使在电压下,凝胶也可以长时间保留酶,并且能够在更便宜的条件下(例如在盐水中)高效地生产氢气。另一个优点是,当您将材料放入水中时,凝胶会自行组装,并沉淀成形成凝胶的纳米纤维。此外,在实验室条件下,酶通过电极通电。因此,没有极端条件的需求。电力可以利用太阳能电池板或风力涡轮机等可再生能源。然而,酶试图避免电荷,因此需要通过化学处理将其固定在适当的位置。但科学家们找到了一种简单有效的方法,将酶附着在电极上并利用它。
如今,环保氢气主要通过电解产生,其中需要贵金属和稀有金属(例如铂)以及水蒸馏来使该过程发挥作用。这使得绿色氢的生产成本几乎是可持续性较差的“灰色”氢的 15 倍。科学家们希望,未来能够在大规模环境中使用这种方法,以降低成本,并实现绿色氢在工业、农业中的大规模使用,并作为清洁能源。
编委会