Aenert. Research Laboratory news
In the endeavour to find new materials to advance energy and industrial production, different approaches are being examined, some of them including completely new technologies, others relying on known materials. Carbenes are one such molecule. They were first discovered in the 1830s when scientists unsuccessfully tried to dehydrate methanol to methylene. But it wasn’t until the 1980s that a stable carbene compound could be synthesised. Carbenes are a group of extremely reactive carbon-based chemicals and have a divalent carbon in their structure which is bound to two adjacent groups by covalent bonds. It has also got two nonbonding electrons which can have parallel (singlet state) or antiparallel spins (triplet state). The preferred state is dependent on the relative energies of both states. If both orbitals are degenerate, the triplet state is favourable. Otherwise, both electrons occupy the orbital lower in energy with antiparallel spins. Until now carbene processes have only been carried out in small batches via test tubes and require expensive chemicals to drive the reaction.
Now (2023), a team of researcher at Lawrence Berkeley National Laboratory (Berkeley Lab) and UC Berkeley has engineered bacteria which can produce new-to-nature carbon products with the potential to provide a powerful route to sustainable biochemicals.
In their research, the scientists used natural products instead of expensive chemical reactants which can be produced by an engineered strain of the bacteria Streptomyces and started a reaction called carbene-transfer reaction. The bacteria use only sugar to produce chemical products through cellular metabolism. Therefore, the carbene chemistry can be performed without toxic solvents or toxic gases typically used in chemical synthesis. This makes the biological process much more environmentally-friendly than the way chemicals are synthesised today.
During experiments, the engineered bacterium was closely observed as it metabolised and converted sugars into the carbene precursor and the alkene substrate. The bacterium also expressed an evolved P450 enzyme. This enzyme used those chemicals to produce cyclopropanes, high-energy molecules which have the potential to be used in the sustainable production of novel bioactive compounds and advanced biofuels. The great achievement of this experiment was that these reactions could now be performed inside the bacterial cell as the cells could produce all of the reagents and cofactors which means that this reaction can be stepped up to very large scales for mass manufacturing.
The chemical reaction consisted of a diazo ester carbene precursor by cellular metabolism and a microbial platform for introducing unnatural carbene-transfer reactions into biosynthesis. The α-diazoester azaserine was synthesised by expressing a biosynthetic gene cluster in Streptomyces albus. The intracellularly produced azaserine served as a carbene donor to cyclopropanate another intracellularly produced molecule—styrene. Catalyst for the reaction were engineered P450 mutants containing a native cofactor with good diastereoselectivity.
Scientists have long tried to create sustainable fuels from bacteria. In 2022, hydrogenation, hydrodeoxygenation and ring opening of biomass-derived furfural was analysed by using Pd/C, Pt/C, Re/C, Ru/C, Rh/C, Ni/C and Cu/C catalysts. Moreover, a generalized micro-kinetic model was developed, which described the kinetics of tested catalysts. The research found that Pd/C could unselectively hydrogenate the furfural ring, aldehyde group or both and was the most active tested catalyst. Selective aldehyde group hydrogenation, followed by deoxygenation was observed with other catalysts. Only Ru/C were found to be able to form methyltetrahydrofuran (45.3% yield) and ring opening products at 200°C. Reaction conditions were optimised in silico for the most active catalysts (Pd, Pt, Re, Ni on carbon). This was achieved by fixing the kinetic parameters obtained by regression analysis and subsequently maximising the yield of the product of interest. Validation experiments confirmed a high Pd/C hydrogenation activity already at 40°C, forming predominantly tetrahydrofurfural (85% yield), while 95% yield of 2-methyltetrahydrofuran was obtained by using a cheap Ni/C at 212°C.
Image: The adsorption of furfural on 0:Cu, 1:Ni, 2:Pd, where A is the side and B the top view. Colour code: C-yellow, H-cyan, O-red, Cu-orange, Ni-dark purple and Pd-light purple
Source: Rok Šivec, Matej Huš, Blaz Likozar, Miha Grilc/ Furfural hydrogenation over Cu, Ni, Pd, Pt, Re, Rh and Ru catalysts: Ab initio modelling of adsorption, desorption and reaction micro-kinetics/ Chemical Engineering Journal 436(11):135070, May 2022/ DOI:10.1016/j.cej.2022.135070/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2023, scientists designed a novel and reusable heterogeneous catalyst, Pd-PdO/ZnSO4 with 1.1 mol% palladium (Pd), for the production of furfural by flash pyrolysis of lignocelluloses at 400°C. For both dry and wet C6 cellulose and its monomers, the furfural yields reached 74–82 mol%, and 96 mol% from C5 xylan and 23–33 wt% from sugarcane bagasse and corncob. The catalyst consisted of a ZnSO4 support for dehydrating and isomerisating of glucose, and a local core-shell configuration for metallic Pd⁰ encapsulated by an oxide (PdO) layer. The PdO layer was active for the Grob fragmentation of formaldehyde (HCHO) from glucose. This was then in-situ steam reformed into syn-gas (i.e. H2 and CO), whereas the Pd⁰ core was actively promoting the last dehydration step for the formation of furfural.
Image: TEM observation of 1.1 mol% Pd-doped ZnSO4 catalyst Panel a for the low-magnification TEM picture. Panels b, c for the high-magnification TEM for Pd clusters and individual Pd particles. Panel d for the STEM observation of catalyst and its mapping of individual elements including Zn, S, Pd, O, and the superimposition of Pd and O (i.e., Pd-O)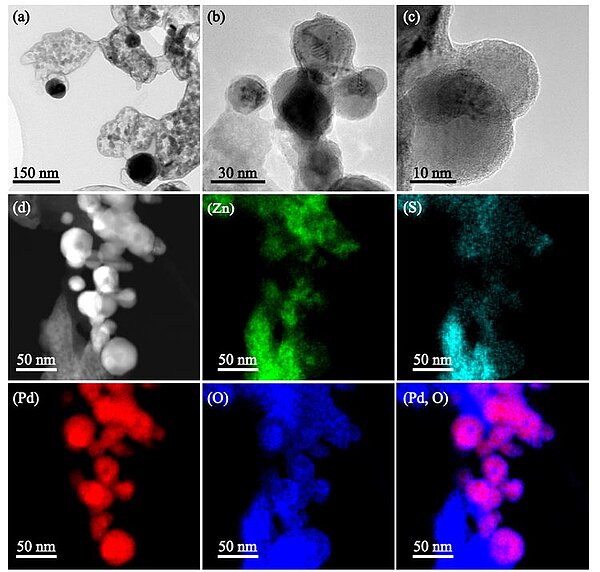
Source: Qiaoqiao Zhou, Jinxing Gu, Jingwei Wang, Anthony De Girolamo/ High production of furfural by flash pyrolysis of C6 sugars and lignocellulose by Pd-PdO/ZnSO4 catalyst/ Nature Communications 14(1), March 2023/ DOI:10.1038/s41467-023-37250-0/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
There are several advantages involved in using bacteria for fuel production: If you use bacteria to synthesise chemicals, it is very likely that carbon emissions during production processes can be reduced dramatically. The study created a scalable, microbial platform for conducting intracellular abiological carbene-transfer reactions to functionalise a range of natural and new-to-nature products and expanded the scope of organic products that can be produced by cellular metabolism.
However, it may still take years before the bacteria are ready for large-scale application. This work will surely inspire others to continue searching for greener, sustainable biomanufacturing solutions.
By the Editorial Board
