Aenert. Research Laboratory news
Over the years, fossil fuels have drawn more and more negative attention owing to the fact that production methods as well as further processing of hydrocarbons release a lot of pollutants into the atmosphere. To mitigate the negative impacts of fossil fuels on the environment, biofuels from renewable sources have been researched extensively in order to provide a suitable alternative to hydrocarbon-derived fuels. One of the main problems, however, is that cost of biofuel production is much higher than fossil fuel production and that it needs subsidies to be a competitive alternative in the market.
Biofuels can be produced by a number of processes, which can be broadly categorized as biochemical and chemical. Biochemical processes utilize proteins called enzymes (biological catalysts) to get a desired product. The research in this field focuses on improving efficiency and quality while reducing negative environmental impacts of the production processes. For example, biodiesel production by enzymatic catalyzed processes has proved to be less energy intensive and more environmentally-friendly. Moreover, the feedstock does not have to be refined in order to be used with a catalyst. Therefore, feedstock such as waste oil can be utilised without having to separate the free fatty acids that can exist in large amounts in the feedstock.
Now (2023), scientists at the Brookhaven National Laboratory have managed to create the structure of an enzyme, AlkB, on an atomic level that can selectively cut carbon-hydrogen bonds and turn simple hydrocarbons into more useful chemicals. The research gives a detailed atomic-level “blueprint” which suggests ways to engineer the enzyme to produce desired products.
The enzyme AlkB was discovered 50 years ago in a machine shop. There, bacteria were found digesting cooling oil and making it smell rancid. When the phenomenon was analysed in an ensuing study, the biochemists found out that the bacterial enzyme AlkB was responsible for the process. Further studies then revealed that the enzyme was partially embedded in the membranes of the bacteria and worked together with two other proteins. But it was not until 2021 that scientists were able to understand the process in its entirety when Brookhaven opened its new cryo-electron microscope (cryo-EM) facility, the Laboratory for BioMolecular Structure (LBMS). This gave them the opportunity to use a cryo-EM on the samples, which has the advantage that it does not need a frozen crystallized sample required for all former x-ray methods, and take pictures of individual frozen protein molecules from many different angles. Afterwards, computational tools analysed the images, identified the common features and generated a high-resolution, three-dimensional map of the enzyme complex. With the help of this map, the scientists were able to put together the known atomic-level structures of the individual amino acids constituting the protein complex to fill in the details in three dimensions.
The detailed picture of the enzyme gave them a clear idea of how AlkB and one of the two associated proteins (AlkG) were able to split the carbon-hydrogen bonds. The solved structure also contained a substrate alkane molecule that was trapped in the enzyme’s active site cavity.
Image: Overall structure
Source: Jin Chai, Gongrui Guo, Sean M. McSweeney, John Shanklin, Qun Liu/ Structural basis for enzymatic terminal C–H bond functionalization of alkanes/ Nature Structural & Molecular Biology volume 30, pages 521–526 (2023), 30 March 2023/ doi.org/10.1038/s41594-023-00958-0/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
Analysing the pictures, the scientists found that it was the position of the alkane with regard to the enzyme’s di-iron center which determined how the activated oxygen interacted with the hydrocarbon. The end of the alkane guided against the activated oxygen would start some chemistry on that last carbon.
Scientists have long tried to improve biofuel catalysts. In 2013, a novel combination for an enzyme-based biofuel cell consisted of a Nafion membrane as an ion transporter that maintained a working cell charge and inhibited membrane degradation. The prototype cell chamber used oxygen (O2) in the cathode cell and glucose in the anode. The Nafion membrane stability had a 0% loss of conductivity. The charge was constant and increased after the addition of glucose. The prototype cell chamber which employed NaCl in the cathode cell and glucose oxidase (GOx) in the anodic chamber was studied for membrane stability. No poisoning from membrane leakage in a controlled pH environment could be detected as well as no crossover at anaerobic operating ambient temperatures and under physiological pH 5 - 7 conditions.
Image: Power output of the MBFC determined over a period of 5 days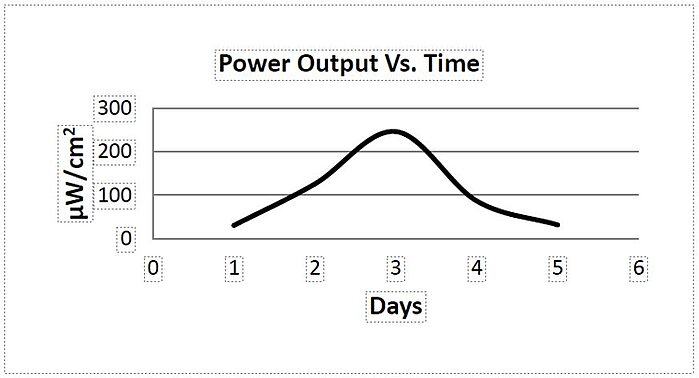
Source: Sivapregasen Gasen Naidoo, Q. Naidoo, Harro von Blottnitz, Guntars Vaivars/ Glucose oxidase as a biocatalytic enzyme-based bio-fuel cell using Nafion membrane limiting crossover/ IOP Conference Series Materials Science and Engineering 49(1), November 2013/ DOI:10.1088/1757-899X/49/1/012062/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 3.0 International (CC BY 3.0)
In 2021, scientists conducted a study of the hydrolytic stability, conductivity and mechanical behaviour of different proton exchange membranes containing sulfonated poly(ether ether ketone) (SPEEK) and sulfonated poly(phenyl sulfone) (SPPSU) ionomers in phosphate buffer solution. The results proved that the membrane stability could be adapted by changing the casting solvent (DMSO, water or ethanol) and procedures, including a crosslinking heat treatment, or by blending the two ionomers.
Image: (a) Structures of sulfonated poly(ether ether ketone) (SPEEK) and sulfonated poly(phenyl sulfone) (SPPSU). (b) Various possible pathways for the reticulation of SPEEK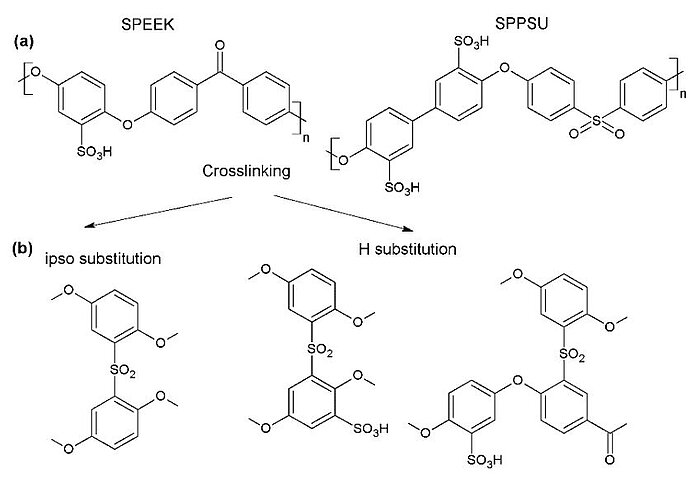
Source: Luca Pasquini, Botagoz Zhakisheva, Emanuela Sgreccia, Riccardo Narducci, Maria Luisa Di Vona and Philippe Knauth/ Stability of Proton Exchange Membranes in Phosphate Buffer for Enzymatic Fuel Cell Application: Hydration, Conductivity and Mechanical Properties/ Polymers 13(3):475, February 2021/ DOI:10.3390/polym13030475/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
The idea of using enzymes for fuel production is attractive because most industrial catalytic processes used for alkane conversions create unwanted byproducts and heat-trapping carbon dioxide (CO2) gas. They also require costly materials and high temperatures and pressure to function efficiently. The biological enzyme, AlkB, on the other hand, operates under more ordinary conditions and with very high specificity. It uses inexpensive earth-abundant iron to initiate the chemistry and produces few byproducts.
The aim of this study is to provide a diverse selection of biocatalysts where you can specifically choose the desired substrate to produce wanted and unique products from abundant hydrocarbons. The method would provide a steerable reaction to convert cheap and abundant alkanes into more valuable bioproducts or chemical precursors, including alcohols, aldehydes, carboxylates, and epoxides.
By the Editorial Board
