The sun is an abundant source of energy. The sunlight which hits the earth in the course of a day is enough to cover the world’s energy consumption for a very long time. This energy can be harnessed using photovoltaic (PV) panels or through mirrors that concentrate solar radiation and used to generate electricity or be stored in batteries or thermal storage. Solar panels, also known as photovoltaics, are made up of semiconductor devices, or solar cells, to convert energy from the sun into electricity.
For this purpose, an electric field is needed for the cells to be able to separate positive charges from negative charges. Therefore, the solar cell gets doped with chemicals so that one layer of the device bears a positive charge and another layer a negative charge. This enables the electrons to flow from the negative side of a device to the positive side which is important for the stability and performance of the device. However, chemical doping and layered synthesis also add extra costly steps in solar cell manufacturing.
Now (2022), scientists at DOE’s Lawrence Berkeley National Laboratory (Berkeley Lab), in collaboration with UC Berkeley, have worked out a method that offers an easier approach to solar cell manufacturing. It consists of forming a perovskite from crystalline solar material with a built-in electric field, which is caused by ferroelectricity. The new ferroelectric material, which was produced in the lab was made of caesium germanium tribromide (CsGeBr3 or CGB). The material was chosen because many of the best-performing halide perovskites naturally contain lead which, according to common opinion, could contaminate the environment and pose a risk to human health during production and after the disposal of the material. Finding a new lead-free halide perovskite formulation which would have the same performance as devices containing lead would be a real game-changer in the industry.
Perovskites crystallize from three different elements and each perovskite crystal has the chemical formula ABX3. Most perovskite solar materials are not ferroelectric because their crystalline atomic structure is symmetrical. There are, however, exceptions. It is these asymmetrical perovskites researchers are looking for as they have ferroelectric potential as they are able to carry an electrical load.
The current research is based on experiments carried out a few years ago, when graduate student researchers at Berkeley were looking for ways to achieve a lead-free ferroelectric perovskite. They suspected that placing a germanium atom in the centre of a perovskite could distort its crystallinity just enough to create ferroelectricity and would free the material of lead. Although the scientists found germanium as a promising material, there were still uncertainties, as finding the right formulation for the perovskite proved difficult.
Continuing their research, the scientists now partnered with the Molecular Foundry and Materials Sciences Division at Berkeley specialising in the design of new materials for a variety of applications, including quantum computing and microelectronics. They used supercomputers at the National Energy Research Scientific Computing Center (NERSC) to perform advanced theoretical calculations based on density-functional theory.
With the help of these calculations, which take atomic structure and chemical species as input to predict properties such as the electronic structure and ferroelectricity, the team discovered CGB, which had all the properties they were looking for: asymmetry and perovskite structure. They relied on the results of their previous research that asymmetrically placing germanium in the centre of the crystal would create a potential that, like an electric field, could separate positive electrons from negative electrons and produce electricity and, to prove this theory, grew tiny nanowires (100 to 1,000 nanometres in diameter) and nanoplates (around 200 to 600 nanometres thick and 10 microns wide) of single-crystalline CGB with great control and precision.
X-ray experiments at the Advanced Light Source highlighted CGB’s asymmetrical crystalline structure as well as a “displaced” atomic structure offset by the germanium centre. A final experiment including photoconductivity measurements found that CGB’s light absorption was tuneable, spanning the spectrum of visible to ultraviolet light (1.6 to 3 electron volts), which was ideal for achieving high energy conversion efficiencies in a solar cell.
For many years, scientists have tried to improve halide perovskites for harnessing sunlight. In 2019, the dynamic surface of Cs–Pb–X nanocrystals was analysed in detail, especially in CsPbX3 perovskites. The studies had two results: For one, the competition between primary alkylammonium and caesium cations for the surface sites during the CsPbX3 nanocrystal nucleation and growth was responsible for the cube/plate shape equilibrium. Short-chain acids and branched amines had an influence on the equilibrium and caused shape-shifting synthesis of pure CsPbX3 cubes, nanoplatelets, nanosheets, or nanowires. Secondly, quaternary ammonium halides were proved to be superior ligands that extended the shelf life of Cs–Pb–X colloidal nanomaterials, improved their photoluminescence quantum yield, and prevented foreign ions from escaping the nanocrystals. This was achieved by combining reduced ligand solubility, due to the branched organic ammonium cation, with the surface-healing capabilities of the halide counterions.
Image: Nanocrystals of different compounds within the Cs–Pb–Br system (a). Side-view of the Cs+ cation substructure shared among the same compounds (b). Thanks to this common structural feature, nanocrystals can be converted one into another by ion trade reactions exchanging PbX2. Since ion trade reactions preserve the nanocrystal backbone, their intermediates are epitaxial heterostructures (c). Transformations between Cs–Pb–Br compounds cause instability issues but provide opportunities for applications as well. One example is the templated conversion of a film of CsPbBr3 emissive nanocrystals into nonemissive Cs4PbBr6 nanocrystals upon exposure to butylamine vapors, which can be reverted by mild heating (d). Reprinted with permission from refs (1,10,13,15,18,and20). Copyright 2020 Royal Society of Chemistry (ref (1)) and 2017–2020 American Chemical Society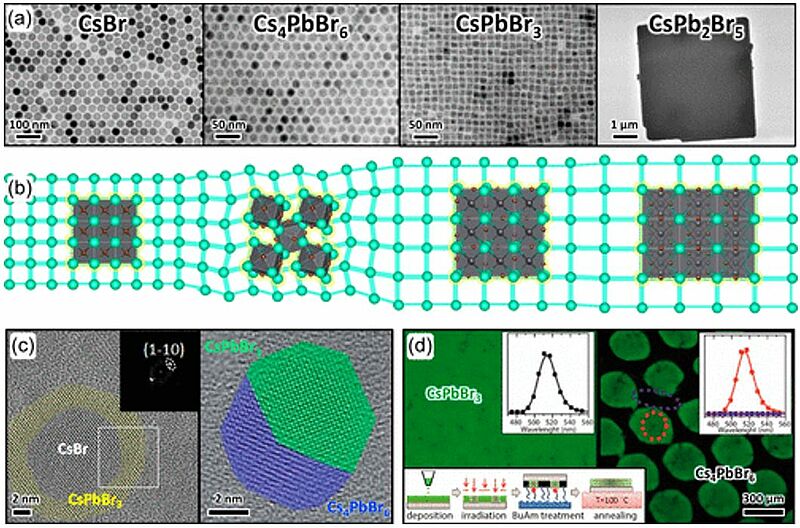
Source: Stefano Toso, Dmitry Baranov, and Liberato Manna/ Metamorphoses of Cesium Lead Halide Nanocrystals/ Acc. Chem. Res. 2021, 54, 3, 498–508, January 7, 2021/ doi.org/10.1021/acs.accounts.0c00710/ Open Source This article is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2022, scientists showed how CsPbCl3 perovskite nanocrystals could be used to drive the phase-selective synthesis of two yet unexplored lead sulfochlorides: Pb3S2Cl2 and Pb4S3Cl2. When homogeneously nucleated in solution, lead sulfochlorides formed Pb3S2Cl2 nanocrystals. On the other hand, the presence of CsPbCl3 started the formation of Pb4S3Cl2/CsPbCl3 epitaxial heterostructures. The phase selectivity was ensured by the continuity of the cationic subnetwork across the interface, a condition not met in a hypothetical Pb3S2Cl2/CsPbCl3 heterostructure. The perovskite domain was then etched, which produced phase-pure Pb4S3Cl2 nanocrystals that could not be synthesized directly.
Image: Halide perovskites as disposable epitaxial templates for the phase-selective synthesis of lead sulfochloride nanocrystals: A solution of PbCl2 reacts with sulfur dissolved in 1-octadecene (S-ODE) to form Pb3S2Cl2 NCs. When reacted with Cs-oleate (R-COO-Cs+) it produces instead CsPbCl3 nanoclusters, that can be further reacted with Pb-oleate, S-ODE, and an alkyl thiol (R-SH) to form Pb4S3Cl2/CsPbCl3 heterostructures. The CsPbCl3 domain can be then selectively etched by the sequential addition of oleylamine (R-NH2), dimethyl formamide (DMF), and oleic acid (R-COOH), yielding colloidally stable Pb4S3Cl2 NCs that could not be obtained by direct synthesis. Atoms color code: Cs = cyan; Pb = grey; S = yellow; Cl = green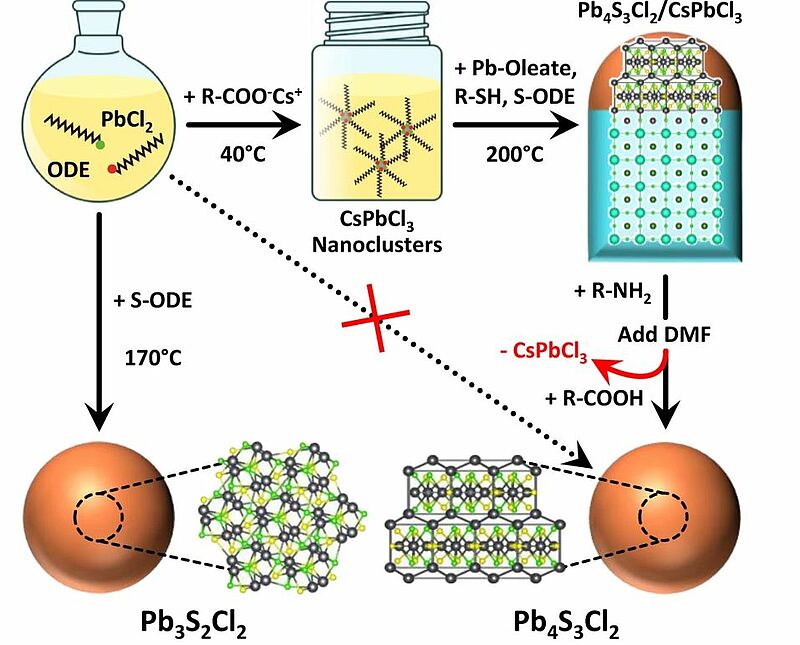
Source: Stefano Toso, Muhammad Imran, Enrico Mugnaioli, Anna Moliterni, Rocco Caliandro, Nadine J. Schrenker, Andrea Pianetti, Juliette Zito, Francesco Zaccaria, Ye Wu, Mauro Gemmi, Cinzia Giannini, Sergio Brovelli, Ivan Infante, Sara Bals & Liberato Manna/ Halide perovskites as disposable epitaxial templates for the phase-selective synthesis of lead sulfochloride nanocrystals/ Nature Communications volume 13, Article number: 3976 (2022), 08 July 2022/ doi.org/10.1038/s41467-022-31699-1/ Open Source This article is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0)
There are many advantages to using lead-free perovskites for harnessing solar energy: they are a solar material which cannot not only harvest energy from the sun but also has property of having a naturally, spontaneously formed electric. CGB could also bring about a new generation of switching devices, sensors, and super-stable memory devices that respond to light. CGB crystals can help save material costs as they are inherently polarized, which means that one side of the crystal builds up positive charges and the other side builds up negative charges, without the need for doping. They are also easy to synthesise compared to silicon.
There is still more research which needs to be carried out before the CGB material can make its debut in a commercial solar device. One of them will include testing the versatile ferroelectric perovskite material, which is essentially a salt, out in the open and see if it works under real-life conditions.