Lemna minor, also called the common duckweed or lesser duckweed, is an aquatic freshwater plant. It is used as animal fodder, bioremediator, for wastewater nutrient recovery, and also biofuel production. Unlike first generation biofuels, duckweed does not require the use of farmland as duckweed is a fairly small aquatic plant that floats on or just below the surface of the water. In nature, duckweed can be found most prevalent in fresh water and wetlands. This allows for a more widespread and accessible production means, and also the dearly required farmland to be used for the production of foodstuff. However, it has an even more important quality as it is able to grow in wastewaters. Wastewaters contain high concentrations of nutrients compared to other bodies of water, such as freshwater or wetlands. Therefore, industrial production of duckweed need not be carried out in freshwater lakes or rivers but could instead be limited to wastewater facilities. Duckweed is also unique in its superior rate of growth and does not require a lot of time to grow. It can effectively double biomass every twenty hours (if under appropriate conditions.) This could yield a large increase in energy supply and a subsequent decrease in energy costs.
Now (2022), scientists at the U.S. Department of Energy’s Brookhaven National Laboratory and collaborators at Cold Spring Harbor Laboratory (CSHL) have modified duckweed so as to produce large amounts of oil. The team added genes to enhance the synthesis of fatty acids, turn those fatty acids into oils, and protect the oil from degradation. Such oil-rich duckweed could be easily harvested to produce biofuels or other bioproducts. The scientists created a strain of duckweed, Lemna japonica, to produce oil at nearly 10 percent of its dry weight biomass. This is seven times higher than soybeans, today’s largest source of biodiesel.
The current project can look back on a long history of duckweed research at Brookhaven Lab research starting in the 1970s. Later, other members of the Biology Department cooperated with the Martienssen group at Cold Spring Harbor to develop a highly efficient method for expressing genes from other species in duckweeds, alongside approaches to suppress expression of the own genes of the plant.
Since Brookhaven researchers had managed to identify the key biochemical factors that are responsible for oil production and accumulation in plants, one goal of the current research was to apply this knowledge and the genetic tools to try and modify plant oil production. Therefore, duckweed was engineered with the genes that control these oil-production factors in order to be able to study their combined effects. One of the oil-production genes identified by the Brookhaven researchers was found to increase the production of the basic building blocks of oil called fatty acids. Another could turn those fatty acids into molecules called triacylglycerols (TAG) consisting of combinations of three fatty acids which can combine and form the hydrocarbons called oils. The third gene produced a protein which coated oil droplets in plant tissues, protecting them from degradation.
Image: Optimization of gene expression in Lemna japonica. (a) TAG contents in N. benthamiana leaves transiently expressing the modified SiOLE with ZmHSP70 intron (SiOLE(*)). (b) Thin layer chromatography (TLC) of lipid from two different P35SL:ZmWRI1b transgenic lines and its parental WT and control line (GFP). (c) Morphology of WT and representative ZmWRI1b overexpression plants. Scale bar=5 mm. (d) Expression and subcellular localization of AtWRI1 with the optimized Anemonia majano CFP fused to the N terminus were assessed by the fluorescence signal of CFP. The CFP fluorescence is shown in blue color and the EYFP is shown in yellow color. The overlapping regions of CFP and EYFP are indicated by purple arrows. Scale bar=40 μm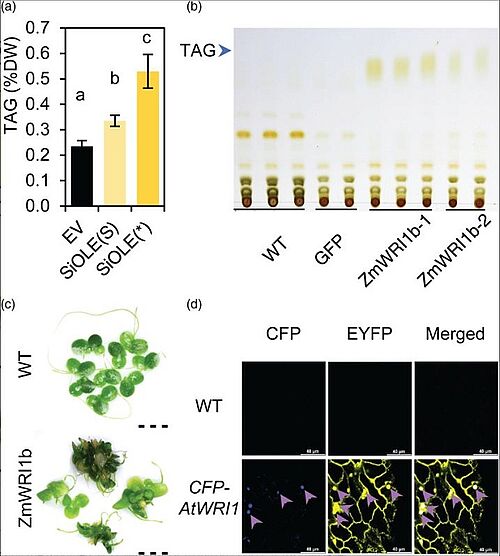
Source: Yuanxue Liang1, Xiao-Hong Yu1, Sanket Anaokar1, Hai Shi1, William B. Dahl2, Yingqi Cai1, Guangbin Luo3, Jin Chai1, Yuanheng Cai1, Almudena Mollá-Morales2, Fredy Altpeter3, Evan Ernst2,4, Jorg Schwender1, Robert A. Martienssen2,4, John Shanklin/ Engineering Triacylglycerol Accumulation in Duckweed (Lemna japonica)/ Plant Biotechnology Journal, 09 October 2022/ doi.org/10.1111/pbi.13943/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
Building preliminary work, the scientists found that increased fatty acid levels triggered by the enhancing gene could have detrimental effects on plant growth. To avoid those effects, the researchers paired that gene with a promoter that can be set to action by adding a small amount of a specific chemical inducer. They created a series of gene combinations to express the improved enhancing, limiting, and protecting factors singly, in pairs, and all together.
Overexpression of each gene modification alone was found to not significantly increase fatty acid levels in Lemna japonica fronds. However, plants engineered with all three modifications increased to 16 percent of their dry weight as fatty acids and 8.7 percent as oil. The best plants accumulated up to 10 percent TAG. Some combinations of two modifications (WD and DO) increased fatty acid content and TAG accumulation dramatically in relation to their individual effects.
These results were also examined under the microscope by producing images of lipid droplets in the fronds of the plants. The duckweed fronds were contacted with a chemical that could bind to oil. The images showed that plants with each two-gene combination (OD, OW, WD) had enhanced accumulation of lipid droplets as opposed to plants with a single gene expression and also when compared to control plants with no genetic modification. Plants from the OD and OWD lines both had large oil droplets, but the OWD line had more of them, producing the strongest signals.
A lot of research has been dedicated to finding alternative biofuel sources to relieve dependency on fossil fuels. In 2014, scientists researched the feasibility of the dual application of duckweed and azolla aquatic plants for wastewater treatment and production of renewable fuels and petrochemicals. The differences in absorption rates of the key wastewater nutrients, ammonium and phosphorus by these aquatic macrophytes were used as the basis for the optimising the composition of wastewater effluents. Analysing pyrolysis products showed that azolla and algae produced a similar range of bio-oils which contained a large spectrum of petrochemicals including straight-chain C10-C21 alkanes, which can be directly used as diesel fuel supplement, or a glycerin-free component of biodiesel. Pyrolysis of duckweed was found to produce a different range of bio-oil components that can potentially be used for the production of “green” gasoline and diesel fuel using existing techniques, such as catalytic hydrodeoxygenation.
Image: Growth of duckweed and azolla in different dilutions of anaerobically digested swine wastewater (ADSW). (A-D) Growing duckweed; (E-G) Growing azolla; (A) and (E) controls; (B) 50% ADSW; (C) 10% ADSW; (D) 2.5% ADSW; (F) 15% ADSW; (G) 2.5% ADSW; bar =1 cm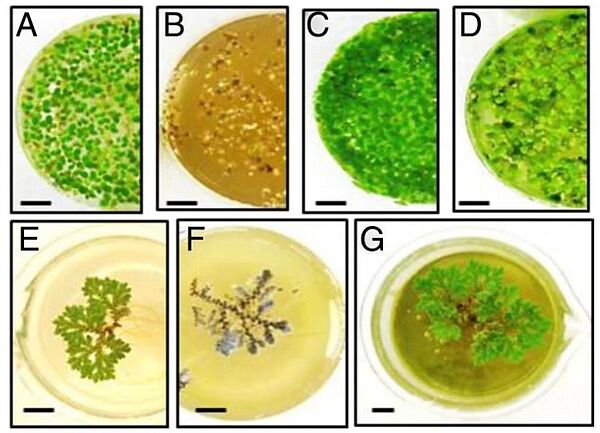
Source: Nazim Muradov, Mohamed Taha, Ana F Miranda, Krishna Kadali, Amit Gujar, Simone Rochfort, Trevor Stevenson, Andrew S Ball & Aidyn Mouradov/ Dual application of duckweed and azolla plants for wastewater treatment and renewable fuels and petrochemicals production/ Biotechnology for Biofuels volume 7, Article number: 30 (2014), 28 February 2014/ doi.org/10.1186/1754-6834-7-30/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution License 2.0 Generic (CC BY 2.0)
In 2014, a Lemna aequinoctialis strain 6000, which has a fast growth rate and the ability to accumulate high levels of starch, was grown in both Schenk & Hildebrandt medium (SH) and in sewage water (SW). The maximum growth rates reached 10.0 g DW m 22 day 21 and 4.3 g DW m 22 day 21, respectively, for the SH and SW cultures, while the starch content reached 39% (w/w) and 34% (w/w). The nitrogen and phosphorus removal rate reached 80% (SH) and 90% (SW) during cultivation, and heavy metal ions assimilation was observed. About 95% (w/w) of glucose was released from duckweed biomass hydrolysates, and then fermented by Angel yeast with ethanol yield of 0.19 g g21(SH) and 0.17 g g21(SW). The amylose/amylopectin ratios of the cultures changed as starch content increased, from 0.252to 0.155 (SH) and from 0.252 to 0.174 (SW). Lemna aequinoctialis strain 6000 could be considered as valuable feedstock for bioethanol production and water resources purification.
Image: Kinetics of duckweed growth in Schenk & Hildebrandt medium (SH) and sewage water (SW). Each data point represents the mean of triplicate values; error bars indicate the standard deviation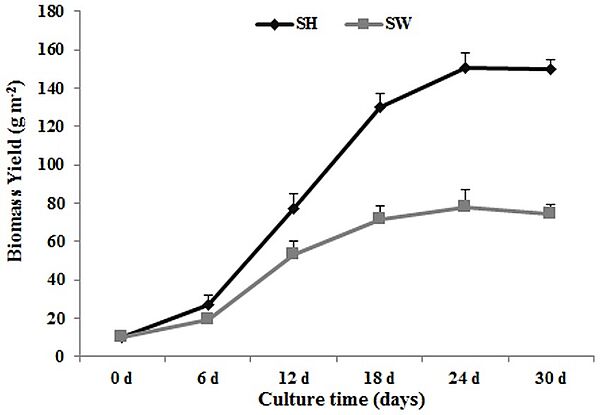
Source: Changjiang Yu, Changjiang Sun, Li Yu, Ming Zhu/ Comparative Analysis of Duckweed Cultivation with Sewage Water and SH Media for Production of Fuel Ethanol/ PLoS ONE 9(12), December 2014/ doi:10.1371/journal.pone.0115023/ Open Access This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
There are several advantages related to producing fuel from duckweed: Duckweed grows fast as it has only tiny stems and roots which is why most of its biomass is in leaf-like fronds that grow on the surface of ponds worldwide. The new engineering approach creates high oil content in all that biomass. Growing and harvesting this engineered duckweed in batches and extracting its oil could be an efficient pathway to renewable and sustainable oil production.
There are also other benefits to be derived from duckweed. As an aquatic plant, oil-producing duckweed wouldn’t compete with food crops for prime agricultural land. It can even grow on runoff from pig and poultry farms. This means this engineered plant could potentially clean up agricultural waste streams as it produces oil.
Future work will focus on testing enhancing, inhibiting and protecting factors from a variety of different sources, optimising the levels of expression of the three oil-inducing genes, and refining the timing of their expression. Moreover, they will be working on how to scale up production from laboratory to industrial levels. This work has several main directions: a) designing large-scale culture vessels for growing the modified plants, b) optimising large-scale growth conditions, and 3) developing methods to efficiently extract oil at high levels.