Hydrogen is increasingly becoming one of the major players in achieving independence from fossil fuels. However, making out the perfect material for storing hydrogen either in gaseous or liquid form has brought along its own set of problems. When in gaseous form, hydrogen requires high-pressure tanks (350–700 bar [5,000–10,000 psi] tank pressure), whereas storage as a liquid needs cryogenic temperatures, as the boiling point of hydrogen at one atmosphere pressure is −252.8°C. Hydrogen can also be stored on the surfaces of solids by adsorption or within solids by absorption. Hydrogen storage is one of the key issues which need solving if we want to advance hydrogen and fuel cell technologies in applications including stationary power, portable power, and transportation. The prospects look promising as hydrogen has a very high energy per mass of any fuel.
Now (2022), scientists at Deakin university have discovered a novel way to separate, store and transport large amounts of gas safely, producing no waste at the same time. The special ingredient to facilitate the process was boron nitride powder. This material can easily absorb substances because it is small. Yet, it has a large amount of surface area for absorption. During the process, the boron nitride powder was fed into a ball mill, which is a type of grinder containing small stainless-steel balls in a chamber, alongside the gases that need to be separated. When the chamber rotates at a high speed, the colliding balls, the powder and the wall of the chamber start a special mechanochemical reaction resulting in gas being absorbed into the powder. As one type of gas is always more quickly absorbed into the powdered material, through separating it out from the others, it can be easily removed from the mill. This process could be repeated over several stages to separate the gases one by one. The ball-milling gas absorption process was found to use up 76.8 KJ/s in order to store and separate 1000L of gases, which was found to be almost 90 per cent less than the energy used in the current separation process applied in the petroleum industry. As soon as the gas was absorbed into the boron nitride, it could be transported safely and easily. Then, when the gas was needed, the powder could be simply heated in a vacuum to release the gas unchanged.
For many years, the problem of efficient hydrogen storage has been at the centre of scientific attention. In 2019, scientists designed the mechanical milling of an alloy consisting of iron (Fe), titanium (Ti) and manganese (Mn) for hydrogen storage in an industrial milling device. The TiFe hydride was used because it was abundant and the constituent elements Ti and Fe were inexpensive, as well as had a high volumetric hydrogen capacity. However, TiFe was difficult to activate, usually requiring a thermal treatment above 400°C. A TiFeMn alloy milled for just 10 min in a 100 L industrial milling device exhibited excellent hydrogen storage properties without any thermal treatment. The TiFeMn alloy did not require activation and showed fast kinetic behavior and good cycling stability. Microstructural and morphological characterisation of the resulting TiFeMn alloys revealed that the material had smaller particle and crystallite sizes, even after a short time of milling. This was found to be responsible for the improved hydrogen absorption-desorption properties.
Image: SEM observations and PSD histograms: A as-received and B as-milled TiFeMn (CM100)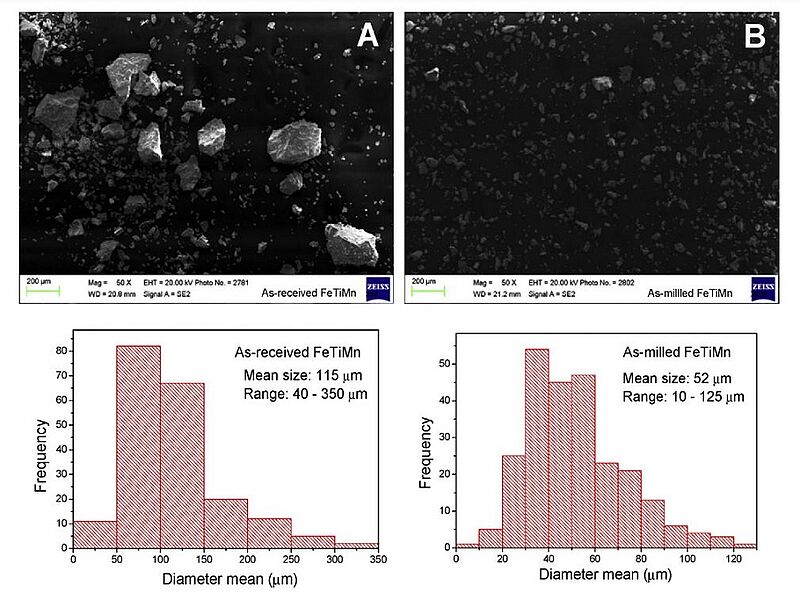
Source: Jose M. Bellosta von Colbe, Julián Puszkiel, Giovanni Capurso, Andreas Franz/ Scale-up of milling in a 100 L device for processing of TiFeMn alloy for hydrogen storage applications: Procedure and characterization/ International Journal of Hydrogen Energy 44(55), February 2019/ DOI:10.1016/j.ijhydene.2019.01.174/ Open Source This article is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0)
In May 2022, scientists designed a thermal activation method for industrially produced titanium-iron-manganese powders (TiFeMn) for hydrogen storage. The influence of temperature and particle size of TiFeMn on the activation process was analysed. The findings suggested that the activation of the TiFeMn material at low temperatures of 50°C was possible and that an increase to 90°C decreased the incubation time for activation: the incubation time of the 90°C/90°C sample was about 0.84 h while ~ 277 h was needed for the 50°C/50°C sample. Selecting TiFeMn particles of larger size, they found, also caused significant improvements in the activation performance of the material. The proposed activation routine made it possible to overcome the oxide layer on the compound surface, which acted as a diffusion barrier for the hydrogen atoms. The activation method caused further cracks and defects in the powder granules, thus producing new surfaces for hydrogen absorption with greater frequency and facilitating to faster sorption kinetics in the subsequent absorption-desorption cycles.
There are several advantages following from the new storage method: The boron nitride powder can be used multiple times for the same gas separation and storage process. There is almost no waste, the process needs no toxic chemicals and leaves no by-products. Boron nitride is classified as a level-0 chemical and is quite safe to store in your house. This, in turn, means that you can store hydrogen in any place and use it whenever needed. It does not require high pressure or low temperatures, so it would offer a much cheaper and safer way to develop things like hydrogen-powered vehicles.
Image: (a) First hydrogen loading of TiFeMn with different particle sizes and (b) corresponding incubation times measured at 40 bar and 70°C. Image: (a) First hydrogen loading of TiFeMn with different particle sizes and (b) corresponding incubation times measured at 40 bar and 70°C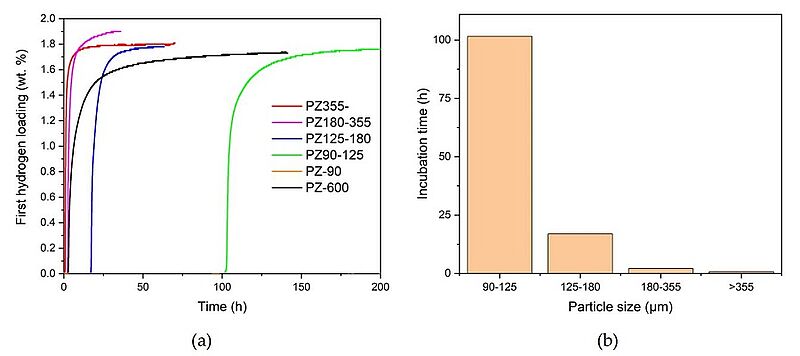
Source: David Dreistadt, Giovanni Capurso, Thu Thi Le, Jose M. Bellosta von Colbe/ An Effective Activation Method for Industrially Produced TiFeMn Powder for Hydrogen Storage/ Journal of Alloys and Compounds, April 2022/ DOI:10.48550/arXiv.2205.09429/ Open Source This article is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International
In the course of their current research, the IFM team has been able to test their process on a small scale, separating about two to three litres of material. However, they hope to gain industry support so that the process can be scaled up to a full pilot. Also, they have submitted a provisional patent application for their process.