The ability of plants to convert sunlight, water and carbon dioxide into carbohydrates and oxygen has fascinated scientists for many years, serving as an inspiration to emulate nature and design similar processes to turn sunlight into sustainable fuels. These processes are summarised under the term artificial synthesis, including, for example, photocatalytic water splitting, and refer to systems which can capture and store the energy received from sunlight in the chemical bonds of a fuel. The growing interest in fuel production through artificial photosynthesis is also reflected in numerous patented inventions, including, for example, US10384195B2 issued by Fujifilm Corporation and Japan Technological Research Association of Artificial Photosynthetic Chemical Process in 2019: Artificial photosynthesis module; or US8519012B2 issued by Climeworks AG in 2012: Artificial photosynthesis.
Now (2022), scientists at Berkeley Lab have created a new device component for artificial photosynthesis which shows outstanding stability and longevity and can selectively convert sunlight and carbon dioxide into ethylene and hydrogen. For the current study, they created a model solar fuels device consisting of a photoelectrochemical (PEC) cell made of copper (I) oxide or cuprous oxide (Cu2O).
Image: Simplified illustration of the PEC/PEC tandem cell configuration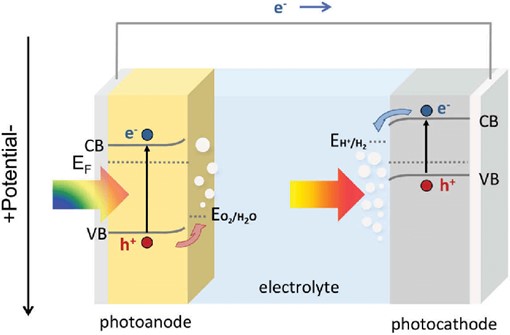
Source: Qi Chen, Guozhen Fan, Hongwei Fu, Zhaosheng Li, Zhigang Zou/ Tandem photoelectrochemical cells for solar water splitting/ Advances in Physics: X Volume 3, 2018 - Issue 1/ DOI:10.1080/23746149.2018.1487267/ Open Access This article is licensed under a Creative Commons Attribution 4.0 International License
Cuprous oxide is one of the most promising materials for artificial synthesis as it is fairly inexpensive and has suitable properties for absorbing visible light. However, its high reactivity to light also causes the material to degrade within a very short time – a problem the scientists knew they had to solve to make the process more reliable. Therefore, they examined the crystal structure of cuprous oxide before and after use. Electron microscopy experiments proved that the stability of the material was very susceptible to light and water. Ensuing X-ray photoelectron spectroscopy (APXPS) revealed that water which contains hydroxide ions caused the material to corrode even faster than other materials. This was because electron-hole pairs separated into electrons and holes to generate charge, but if electricity was not generated, the electrons simply reacted with the material and corroded it.
Therefore, in order to improve the stability of the material they created a cuprous oxide PEC coated with silver on the upper side and gold/iron oxide underneath, which took the shape of a Z scheme and was inspired by the electron transfer in natural photosynthesis. Also, the diversity in materials at the interface could stabilise the system by providing additional electrons to recombine with the holes of the cuprous oxide. Finally, they designed a physical model of the Z-scheme artificial photosynthesis device with an improved a cuprous oxide PEC, which was able to produce ethylene and hydrogen very efficiently.
Artificial photosynthesis systems have received a lot of scientific attention over the past decade. In 2011, for example, scientists created a photocathode for solar H2 production, which consisted of electrodeposited cuprous oxide and was not susceptible to photocathodic decomposition in water through nanolayers of Al-doped zinc oxide and titanium oxide. The system was activated for hydrogen production with electrodeposited Pt nanoparticles. The scientists also analysed the characteristics of the different surface protection components and found that in many cases electrodes showed photocurrents of up to −7.6 mA cm−2 at a potential of 0 V. The electrodes remained active after 1 h of testing, cuprous oxide was found to be stable during the water reduction reaction.
Image: Schematic diagram for photocatalytic water splitting using a Z-scheme with an aqueous redox mediator: (1) light absorption, (2) electron excitation from the VB to the CB, (3) electron(s) transfer to the metal cocatalyst for H + reduction to produce H2, (4) hole(s) transfer to the surface to react with redox mediator, (5) electron(s) transfer to the surface to react with redox mediator and (6) hole transfer to metal oxide cocatalyst to catalyse water oxidation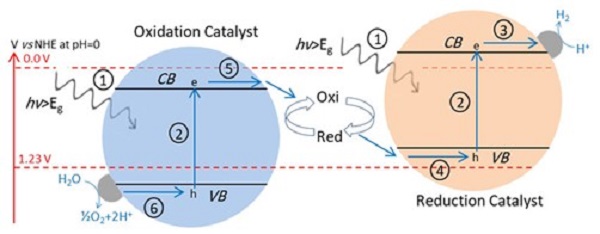
Source: Muhammad Amtiaz Nadeem, Ahmed Abdeslam Ziani, Mohd Adnan Khan, Hicham Idriss/ An Overview of the Photocatalytic Water Splitting over Suspended Particles/ Catalysts 11(1):60, January 2021/ DOI:10.3390/catal11010060/ Open Access This article is licensed under a Creative Commons Attribution 4.0 International
In 2018, a photocathode with a coaxial nanowire structure consisting of a Cu2O/Ga2O3-buried p–n junction was engineered that could achieve efficient light harvesting to over 600 nm, reaching a total hydrogen generation capacity of nearly 80%. The electrode had a photocurrent onset over +1 V and a photocurrent density of ~10 mA cm−2 at 0 V. Through conformal coating via atomic-layer deposition of a TiO2 protection layer stable operation of over more than 100 h was achieved. With the help of NiMo as the hydrogen evolution catalyst, a Cu2O photocathode was achieved with stable operation in a weak alkaline electrolyte. To prove the efficiency of the cathode, the scientists built an all-oxide unassisted solar water splitting tandem device which had about 3% solar-to-hydrogen conversion efficiency.
Using cuprous oxide as a material for artificial synthesis has enabled the scientists at Berkeley Lab to visibly improve the conversion process in terms of efficiency, but also in terms of cost. This is because the material is relatively inexpensive and has suitable properties for absorbing visible light. Also, researching and understanding the functioning of the material in a photosynthesis system gave the scientists the opportunity to design more durable methods and reduce waste.
The next stages of their research will involve developing new solar fuel devices for liquid fuel production applying their newly-gained insights. This will hopefully eventually lead to prolonged system activity as well as less maintenance.