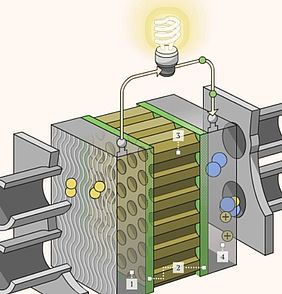
The synthesis of catalysts with flexible properties and sizes has made a big step forward thanks to new nanotechnologies which can help tune the catalytic activity to suit the requirements of the chemical reaction. Scientists have long been researching new selective catalysis with solution-dispersed or supported-metal nanocatalysts. However, several challenges remain, particularly concerning the structural stability of the catalytic active phase.
Core-shell nanostructures consist of nanoparticles which are protected by an outer shell that prevents their migration and coalescence during the catalytic reactions. Researchers at the US Department of Energy’s (DOE) Brookhaven National Laboratory and the University of Arkansas have now (2019) managed to improve the structure of these catalysts further and designed a highly efficient catalyst for extracting electrical energy from ethanol. The ternary Au@PtIr core-shell catalyst can release the full potential of ethanol by means of electrooxidation.
To make the catalyst, scientists developed a synthesis method that placed platinum and iridium on gold nanoparticles. The platinum and iridium particles formed “monoatomic islands” across the surface of the gold nanoparticles, which was responsible for the outstanding performance of the catalyst, as the gold particles increase the ability of the platinum-iridium monoatomic islands to cleave carbon bonds.
Each ethanol molecule can release 12 electrons (12e) by means of electrooxidation. This process involves cleavage of the C-C bonds and several dehydrogenation and oxidation steps. The new catalyst showed that breaking those bonds at the right time was important when trying to unlock the energy stored within the hydrocarbon atoms.
Formerly, this reaction resulted in an incomplete oxidation, as the catalysts did not break all of the carbon-carbon bonds and released fewer electrons. They also split the hydrogen atoms early in the process, which generated carbon monoxide and poisoned the catalysts’ ability to function over time. To complete a 12-electron full oxidation of ethanol, the carbon-carbon bond has to be broken at the beginning of the process while hydrogen atoms are still attached because hydrogen inhibits the formation of carbon monoxide.
Research on core-shell electrocatalysts can look back on a long history of research. In 2013, a team of scientists carried out research on a highly active and stable core-shell/C catalyst and a novel synthetic strategy for its preparation. During the experiment a catalyst with a Pd3Cu1/C core and a selective Pt shell was created using a Hantzsch ester as a reducing agent. A Pd@Pd4Ir6/C catalyst was also designed and synthesized for the hydrogen oxidation reaction. The developed catalysts showed high activity, high selectivity, and 4,000 hours of long-term durability.
In 2017, scientists conducted research on a highly conductive nanoporous architecture of an iridium oxide shell on a metallic iridium core, created through the fast dealloying of osmium from an Ir25Os75 alloy, which showed high oxygen evolution activity as well as stability. It had an improved activity-stability factor compared to conventional iridium-based oxide materials. The study concluded that the activity-stability factor was a key metric for producing efficient oxide-based anodic water electrolyzer catalysts.
The main disadvantage of the former core-shell catalysts was that the efficiency of the combined materials was not as high as in the new catalyst. They were not able to break all of the carbon bonds and thus fewer electrons were released. Also, due to an early dismantling of the hydrogen atoms, the carbon atoms were exposed to the formation of carbon monoxide, which poisoned the catalyst.
The new catalyst with its unique core-shell structure speeds up the steps of dehydrogenation and oxidation which are needed to release the energy stored in ethanol. The gold nanoparticle cores induce tensile strain in the platinum-iridium mono-atomic islands, which increases their ability to break the carbon bonds, and then they strip away the hydrogen atoms. Compared to former catalysts, the new catalyst breaks the C-C bonds at the right time. This process is a game-changing discovery that will enable the use of ethanol fuel cells as an effective source of ‘off-grid’ electrical power, especially for liquid fuel-cell-powered drones.
The next step, the scientists explain, is to build devices that incorporate the new catalyst. The discoveries made by this study could also help guide the rational design of future multicomponent catalysts for other applications.