In view of the current endeavours to reduce fossil fuel dependence and facilitate the transition to more sustainable ways of fuel production, in recent times biogas has been afforded increased attention by scientists around the world. Biogas plants use bacteria to break down biomass in an oxygen-free environment to form biogas. This gas usually contains almost 60 percent of methane and more than 40 percent of CO2. Biogas can be used to generate electricity and heat in combined heat and power units or upgraded to natural gas and transferred to the natural gas network. Concerning the CO2, a waste product of the process, not effective method has so far been implemented to utilise its potential.
Now (2022), scientists at the Fraunhofer Institute for Microengineering and Microsystems IMM have now found a method to convert CO2 into additional methane and thus increase the methane yield from biogas plants. The process has already been successfully tested in the course of a first project stage and the research team is currently trying to increase the output the demonstration plant to five cubic meters of methane per hour.
During the first project stage, the scientists constructed a demonstration plant which could convert one cubic metre of biogas per hour into one cubic metre of methane with a thermal power equivalent of ten kilowatts of the electrolyser required to produce the hydrogen for the process. In the follow-up project, the researchers are now trying to scale up the demonstration plant by five times, which corresponds to a thermal output of 50 kilowatts. One of the greatest challenges of the process is that the amount of energy which can be harnessed from wind and photovoltaic systems underlies significant fluctuations. Therefore, the scientists want to modify the system so that it can respond to fluctuations in energy quickly. Storing hydrogen would be technically feasible but complicated and therefore expensive.
The second challenge of the project pertains to developing efficient catalysts. The scientists decided to use a microcoating consisting of precious metals. In the process the hydrogen and the CO2 will flow through a large number of microchannels coated with the catalyst and react with each other. This helps increase the contact surface between the gases and the catalyst material and reduces the amount of catalyst required. Also, several microstructures of this kind are stacked on top of each other in the reactor which increases its output.
Converting CO2 into energy has been of interest to scientists around the globe for quite some time. In 2020, scientists performed catalytic deoxygenation of coconut oil in a continuous-flow reactor over bimetallic NiCo/silicoaluminophosphate-11 (SAPO-11) nanocatalysts, a medium-pore silicoaluminophosphate molecular sieve with tunable acidity, for hydrocarbon fuel production. The scientists analysed the conversion and product distribution efficiency over NiCo/SAPO-11 with different applied co-reactants, including water (H2O) or glycerol solution, performed under nitrogen (N2) atmosphere. The hydrogen-containing co-reactants were used as in-situ hydrogen sources for the deoxygenation. The results showed that applying co-reactants to the reaction enhanced the oil conversion. The main products formed under the existence of H2O or glycerol solution were free fatty acids and their corresponding Cn−1 alkanes. The addition of H2O increases the triglyceride breakdown into free fatty acids, whereas the glycerol donates hydrogen which can initiate hydrogenolysis of triglycerides and leads to a greater amount of free fatty acids. As a result, the free fatty acids can be deoxygenated to their corresponding Cn−1 alkanes and show that the NiCo/SAPO-11 has the capability to produce hydrocarbon fuels even in the absence of external H2 source.
Image: (a) Scanning electron microscopy (SEM) image; (b) transmission electron microscopy (TEM) image; (c) and (d) corresponding energy dispersive X-ray spectrometer (EDS) elemental mappings of Ni and Co; (e) and (f) EDS line profile of the NiCo/SAPO-11 catalyst.
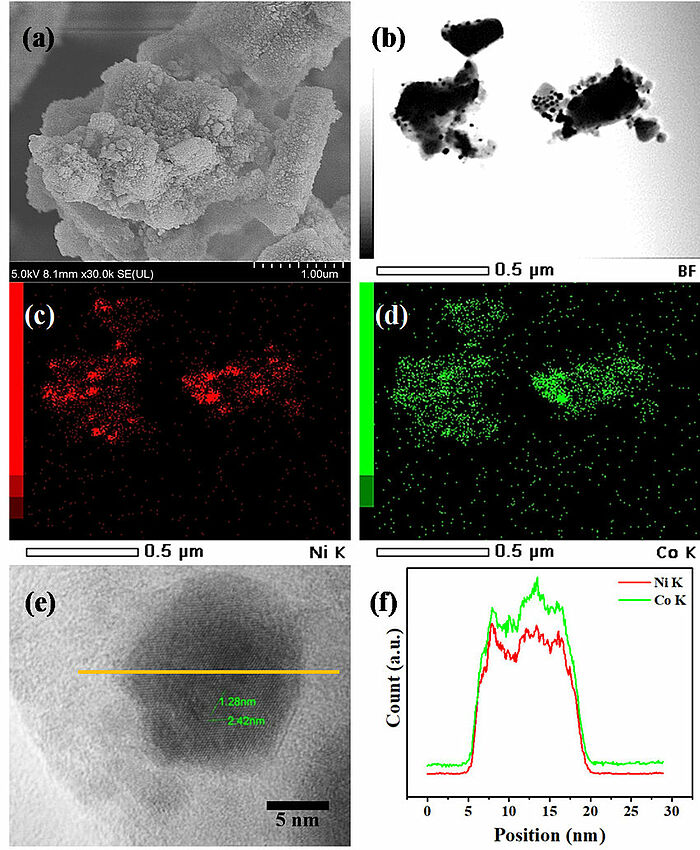
Source: Jeeranan Nonkumwong, Thongthai Witoon, Navadol Laosiripojana/ Effect of Water and Glycerol in Deoxygenation of Coconut Oil over Bimetallic NiCo/SAPO-11 Nanocatalyst under N2 Atmosphere/ Nanomaterials 10(12):2548, December 2020/ DOI:10.3390/nano10122548/ Open Source This article is licensed under a Creative Commons Attribution 4.0 International
In 2022, scientists conducted high-temperature water reactions to reduce carbon dioxide by using an organic reductant and a series of metals and metal oxides as catalysts, as well as activated carbon (C). The CO2 source employed consisted of sodium bicarbonate and ammonium carbamate. Glucose was the reductant. Cu, Ni, Pd/C 5%, Ru/C 5%, C, Fe2O3 and Fe3O4 were the catalysts tested. The products received through CO2 reduction including formic acid and other subproducts from sugar hydrolysis including acetic acid and lactic acid. Higher yields of formic acid (53% and 52%) were achieved by using C and Fe3O4 as catalysts and sodium bicarbonate as carbon source. Reactions with ammonium carbamate yielded an amount of formic acid up to 25% by using Fe3O4 as catalyst. Depending on the catalyst, the fraction of formic acid coming from the reduction of the isotope of sodium bicarbonate varied from 32 to 81%.
Image: SEM images of the catalyst particles
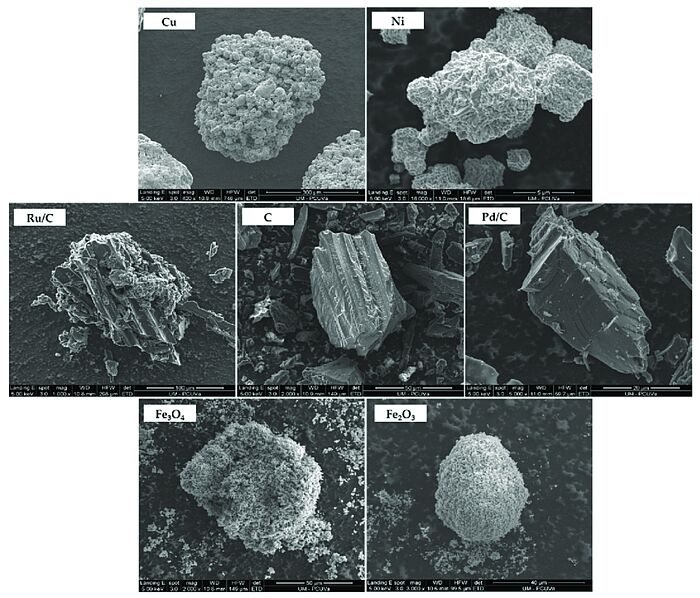
Source: Maira I. Chinchilla, Fidel A. Mato, Ángel Martín, María Dolores Bermejo/ Hydrothermal CO2 Reduction by Glucose as Reducing Agent and Metals and Metal Oxides as Catalysts/ Molecules 27(5):1652, March 2022/ DOI:10.3390/molecules27051652/ Open Source This article is licensed under a Creative Commons Attribution 4.0 International
The new comprehensive biogas plant utilisation has several potential advantages: firstly, a greater amount of biogas can be produced and utilised, which far exceeds the 60 percent yielded by former processes. This has been enabled by the chemical reaction of CO2 conversion, which was discovered more than a hundred years ago, but could not be integrated into the biogas production process until now. However, in view of the necessary energy transition, utilising CO2 will surely make a decisive contribution to achieving a more sustainable environment.
In the current project, the scientists are focusing their attentions on implementing a larger demonstrator and realizing dynamic operation. They plan to put their reactor into operation by 2023 so they can test it under real conditions at a biogas plant. The final goal is to scale production up to 500 kilowatts by 2025 and again to one to two megawatts by 2026.
Energy News Monitoring
Method for Increased Methane Yield from Biogas Plant
