Hydrogen is considered to be one of the most important resources for the energy transition as it can be used as a transport fuel but also energise industrial processes. However, there are still several obstacles, which inhibit the widespread application of hydrogen. One of them is that hydrogen is not easy to transport. A possible solution to this problem is to convert hydrogen into methanol because methanol can be transported and stored more easily. The CO2 needed for conversion of hydrogen into methanol could be taken directly from the atmosphere or from industrial processes where it is often generated as a by-product. Methanol can then be converted back into hydrogen and CO2 with the help of a methanol reformer. What sounds like an easy to perform process brings along its own set of problems, including, for example, catalysts which are needed to set the reaction going. They are often made of copper-zinc-oxide powder which is compressed to extruded pellets and added to the reactor. However, the movements, which occur in mobile applications, can cause catalyst attrition which contaminates the fuel cell. Another problem is that the catalyst material is not fully utilised, and the reaction rate is low owing to relatively low reaction temperatures. Also challenging can be the heat management as the reactor needs constant heat to keep the steam reforming reaction going.
George Olah Renewable Methanol Plant, Iceland
Now (2022), scientists at Fraunhofer Institute have started a project dedicated to improving the design of methanol reformers so that they are able to overcome some of the aforementioned challenges. The most prominent amongst them is, of course, catalyst efficiency which the scientists tried to improve in the first phase of the project. For this purpose, they used catalyst coatings comprising precious metals, which do not abrade and have similar properties as the catalytic converter in a car. Moreover, they modified the makeup of the reformer. They began by coating plate heat exchangers with the catalyst material and stacking them up to 200 plates high. When the gas passes through them, it gets into contact with the catalyst and is heated in the narrow channels. Additionally, the researchers included the use of waste heat in the system, which resulted in high overall efficiency rates.
Currently, the team of researchers are working on a prototype reformer with 35 kilowatts of power which they plan to complete by the middle of 2022. However, this is not the only reformer they are developing: in the course of this long-term project, they intend to integrate various prototypes into land vehicles to test them. For maritime applications, for example, the researchers are also designing a reformer providing 100 kilowatts of power. In the long run, they might even be able to produce reformers from lighter materials such as titanium instead of steel, which might play a crucial role when introducing the technology into automotive applications etc. as it can reduce weight and energy consumption.
Easy hydrogen storage has been of great interest to scientists for quite some time. In 2017, for example, a strategy to transform methanol to hydrogen for at least 32 hours at near-room temperature was developed. The strategy consisted of two main procedures: methanol transformed to formic acid transformed to hydrogen and methanol transformed to nicotinamide adenine dinucleotide (NAD) + hydrogen (H) and finally to hydrogen. Formic acid and the reduced form of nicotinamide adenine dinucleotide (NADH) were simultaneously produced by means of the dehydrogenation of methanol through the cooperation of alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH). Then, formic acid was transformed to hydrogen by a new iridium polymer complex catalyst.
Image: General strategy (a) and detailed reaction system (b) for the generation of H2 from methanol at near-room temperature. Black arrows indicate the first procedure (CH3OH → HCOOH → H2). Red arrows indicate the second procedure (CH3OH → NADH → H2).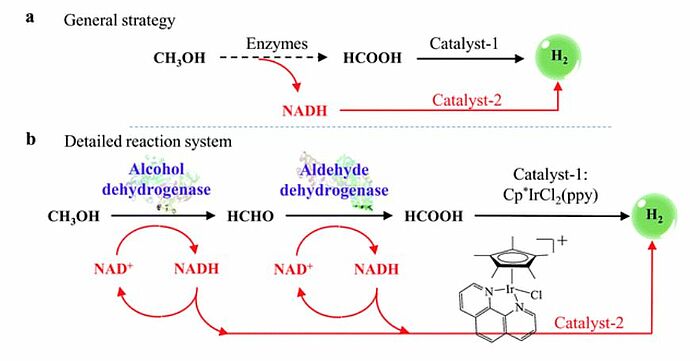
Source: Gui-Liang Xu, Xiang Liu, Xinwei Zhou, Chen Zhao, Inhui Hwang, Amine Daali, Zhenzhen Yang, Yang Ren, Cheng-Jun Sun, Zonghai Chen, Yuzi Liu & Khalil Amine/ Hydrogen generation from methanol at near-room temperature/ Chemical Science Issue 11, 2017/ doi.org/10.1039/C7SC01778B/ Open Access This article is licensed under a Attribution 4.0 International (CC BY 4.0)
An enzyme mimic was used to convert nicotinamide adenine dinucleotide (NAD) + hydrogen (H) to hydrogen and nicotinamide adenine dinucleotide (NAD+). Nicotinamide adenine dinucleotide (NAD+) was then reconverted to nicotinamide adenine dinucleotide (NAD) + hydrogen (H) by repeating the dehydrogenation of methanol. The strategy and the catalysts used in this research could be used for hydrogen production from other organic molecules (e.g. ethanol) or biomass (e.g. glucose).
In 2021, a process integration framework based on an energy mix system was designed to simultaneously produce methanol and H2. The computer software Aspen Plus was used to develop two process models as well as perform a techno-economic assessment. Process 1 was considered the base case process: through the coal–biomass gasification process synthesis gas was produced, which was then further converted into H2 and methanol. Conversely, in the new process 2 design the coal–biomass gasification technology in process 1 was integrated into the methane reforming technology in order to reduce energy loss while increasing net fuel production. For the technical analysis, the scientists compared the fuel production rates, carbon conversion efficiencies and specific energy requirements for both models. The results showed that the process 2 design had higher methanol and H2 production rates while energy consumption was lower. Additionally, the specific energy requirement for process 2 was 29% lower compared to the process 1 design, leading to an increase in the process efficiency of case 2 by 3.5%.
Image: Process model for converting coal and biomass to methanol and hydrogen.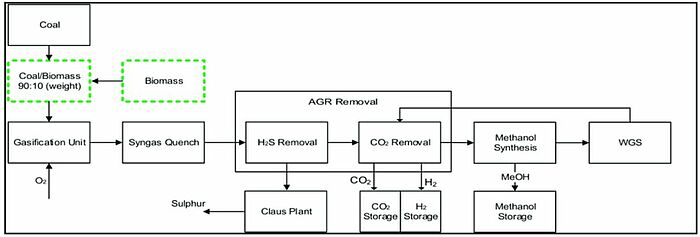
Source: Usama Ahmed, Umer Zahid, Sagheer A. Onaizi, Abdul Gani Abdul Jameel/ Co-Production of Hydrogen and Methanol Using Fuel Mix Systems: Technical and Economic Assessment/ Applied Sciences 11(14):6577, July 2021/ DOI:10.3390/app11146577/ Open Access This article is licensed under a Attribution 4.0 International (CC BY 4.0)
One of the advantages of the reformers is that they need a lot less space, only around 17% of the space a conventional reformer would need, which is an important feature for mobile applications. The researchers have also improved the catalyst technology by using coatings containing precious metals similar to those found in automotive catalytic converters because they show less attrition. Therefore, less catalyst material is required. As the catalyst materials also have a higher activity, the amount of catalyst needed is reduced even further, and consequently also the costs. Moreover, the catalyst does not produce any by-products, such as carbon monoxide.
The ultimate goal of the research team is to design reactors which are suitable for large-scale hydrogen production and also use processes which are very similar to those already established for other energy processes. They are confident that their research will make a contribution to providing a readily-available and affordable power source.
