Aenert. Research Laboratory news
Hydrogen is an abundant material constituting about 75 per cent of the mass of the universe. It is also an element that will certainly one day be used on a larger scale for vehicle propulsion. In order to achieve this goal, improving storage methods is of key importance as efficiently storing this material is a challenging task and has to fulfil certain parameters in order to conform to safety norms. Hydrogen has a high energy per mass, but low energy per unit volume due to its low ambient temperature. It can be stored physically as a gas or a liquid. Storage in the form of gas requires high pressure tanks. If it is stored as a liquid, on the other hand, it needs cryogenic temperatures since the boiling point of hydrogen is −252.8°C. It can also be stored on the surfaces of solids or within solids. All storage options which are available have one thing in common: they need large storage tanks. This is not so much of a problem if stationary applications are concerned. However, when it comes to fuel-cell powered mobility, enabling a range of 200-300 kilometres while providing safe and easy re-fuelling becomes more difficult.
Recently (2021), scientists at Fraunhofer have designed a hydrogen-based fuel which is suitable for small vehicles. The fuel is based on solid magnesium hydride and called POWERPASTE. POWERPASTE was designed in such manner that it could store hydrogen in a chemical form at room temperature and atmospheric pressure to be then released on demand. Also, the scientists made sure that POWERPASTE would only begin to decompose at temperatures of around 250°C, which means that it does not self-ignite even when an e-scooter is exposed to the sun for hours. Moreover, re-fuelling is extremely simple: no filling stations are required, motorists merely have to replace an empty cartridge with a new one and then refill the tank with mains water either at home or underway.
The starting material of POWERPASTE was magnesium, an abundant element and a readily-available raw material. Magnesium powder was mixed with hydrogen to form magnesium hydride in a process conducted at 350°C and five to six times atmospheric pressure. Then the scientists then added an ester and a metal salt in order to form the finished product. Installed in a vehicle, the POWERPASTE was released from a cartridge by means of a plunger. By adding water from an onboard tank, the ensuing reaction generated hydrogen gas in a quantity corresponding to the actual requirements of the fuel cell. Another beneficial feature was that only half of the hydrogen came from the POWERPASTE, the rest was produced by the added water, which added to the energy storage density of the product.
Scientists have long tried to improve hydrogen-propelled engines. In 2019, a lightweight and flexible fuel cell based on paper substrate and Al foil was developed, which was used as an in-situ hydrogen source by reaction with an electrolyte solution during operation. Owing to the inhibited hydroxyl transportation by the porous cellulose network, the strong Al corrosion reaction could be controlled, in spite of the strong alkaline electrolyte being used, so that the fuel cell could be discharged for more than 5 hours at 0.83 V. The faradaic and energy efficiencies were as high as 72%. The fuel cell flexibility was also quite good when facing different bending angles. In view of its moderate power output, this flexible paper-based hydrogen fuel cell was suitable for powering various low-wattage and flexible devices, such as wearable electronics. However, higher power could be obtained by suitable stacking of the fuel cells.
Image: Schematic diagrams of the flexible hydrogen PBFC: (a) Working principle; (b) Exploded view of the cell structure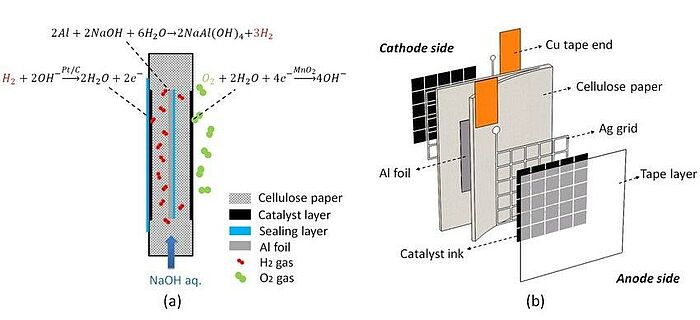
Source: Yifei Wang, Holly Kwok, Yingguang Zhang, Wending Pan/ Flexible hydrogen fuel cell fabricated on paper with embedded aluminium foil/ E3S Web of Conferences 83:01004, January 2019/ DOI:10.1051/e3sconf/20198301004/ Open Source This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2022, a team of scientists prepared Nafion membranes for hydrogen synthesis from methanol which comprised two different nanocomposite MF-4SC membranes and was modified with polyaniline (PANI) through the casting method in two different polyaniline infiltration procedures. Operating conditions were improved concerning current density, stability, and methanol concentration. The cell performance of various parameters, such as methanol concentration, water, and cell voltage were analysed. The energy required for pure water electrolysis was evaluated at different temperatures for the different membranes. PEM electrolysers were shown to provide better hydrogen production of 30 mL/min, working at 160 mA/cm2. Methanol–water electrolysis was found to require less electrical power than pure water electrolysis. The power consumption in methanol electrolysis amounted to 2.34 kW h/kg of hydrogen. This was several times lower than the electrical energy needed to produce 1 kg of hydrogen by water electrolysis.
Image: SEM images of the surface of the MF-4SC membrane (a) and composite membrane MF-4SC/PANI-3H* (b). SEM images of the cross section of an MF-4SC membrane (c) and composite membrane MF-4SC/PANI-3H* (d)
Source: Carlos Sanchez, Francisco J. Espinos, Arturo Barjola, Jorge Escorihuela/ Hydrogen Production from Methanol–Water Solution and Pure Water Electrolysis Using Nanocomposite Perfluorinated Sulfocationic Membranes Modified by Polyaniline/ Polymers 14(21):4500, October 2022/ DOI:10.3390/polym14214500/ Open Source This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
Power paste is an advantageous invention for several reasons: Firstly, it has a high energy storage density which is substantially higher than that of a 700-bar high-pressure tank. And compared to batteries, it has ten times the energy storage density. Its high energy storage density also makes it a suitable fuel for cars, delivery vehicles and range extenders in battery-powered electric vehicles. POWERPASTE could also significantly extend the flight time of large drones, which would make it especially useful for survey work, such as the inspection of forestry or power lines. In addition to providing a high operating range, the paste does not require an expensive infrastructure. If there are no hydrogen stations, filling stations could simply sell it in cartridges or canisters instead, as the paste is fluid and pumpable. At first, such filling stations could supply smaller quantities of POWERPASTE, contained in a metal drum, for example, and then expand in line with demand. This would require a capital expenditure which is significantly lower than for traditional hydrogen storage. The paste is also cheap to transport, since neither costly high-pressure tanks are involved nor the use of extremely cold liquid hydrogen.
Fraunhofer IFAM is currently building a production plant for POWERPASTE at the Fraunhofer Project Center for Energy Storage and Systems ZESS. This plant is scheduled to start operating in 2021 and will be able to produce up to four tons of it a year – not only for e-scooters.
By the Editorial Board