Grid-connected energy storing systems will play an increasingly important role in the near future, as intermittent renewable energy sources are expanding and the demand for improved power quality and energy management is rising. One of the most promising technologies in this respect is electrochemical storage systems because they are flexible, efficient, and scalable. Among them redox flow batteries have high potential and many advantages, such as scalable sizing, high efficiency, room temperature operation, and a long charge/discharge life. The most-marketed redox flow batteries are all-vanadium redox batteries. However, despite their wide application, such batteries remain a mystery when it comes to diagnosing performance.
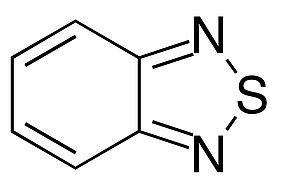
Now (2021), scientists at Argonne National Laboratory have discovered that fluorescence might be able to reveal what processes are active within flow batteries when they operate. In a study, researchers designed carbon-based redoxmer molecules that could both carry energy within the battery and also signal a problem called crossover – redoxmers migrating to the wrong side of the battery. This is a particularly challenging task, as it concerns very small molecules dissolved in an electrolyte and porous membranes. When crossover occurs and the redoxmers penetrate the membrane between the two tanks, it can degrade the performance of a battery. The research team designed two chemical variations of a common anode (negative)-side redoxmer, 2,1,3-benzothiadiazole (BzNSN). BzNSN is stable during charging, is easily dissolved in a solvent-based electrolyte and can be manipulated to fluoresce under ultraviolet light. These properties made it a very suitable candidate for a self-reporting agent on battery health within some types of flow batteries. To measure the fluorescence of the molecules in common flow battery fluids, the researchers relied on fluorimetry measurements, which showed different behaviour depending on the electrolyte salts used. Electrochemical stability measurements also showed that one molecular design in particular maintained its electrochemical function and stability over days when in its charged state. In a separate experiment, the tell-tale fluorescent glow under ultraviolet light was used to detect crossover of the redoxmer in real time, which revealed how the redoxmer molecule movements change depending on the electrolyte composition.
Problems with crossover processes in redox flow batteries have been at the centre of scientific interest for some time. In 2017, scientists demonstrated that active-species crossover ceased when adapting the pore size of the membrane to molecular dimensions and increasing the size of the active material above the pore-size exclusion limit. When oligomeric redox-active organics (RAOs) were paired with microporous polymer membranes, the rate of active-material crossover was reduced more than 9000-fold compared to traditional separators at minimal cost to ionic conductivity. This corresponded to an absolute rate of RAO crossover of less than 3 μmol cm-2 day-1 (for a 1.0 m concentration gradient), which exceeded performance targets recently set forth by the battery industry. This strategy was common to both high and low-potential RAOs in a variety of non-aqueous electrolytes.
In 2019, a study looked at state of charge estimation from open cell voltage measured currentless at a reference cell as well as from open circuit potentials measured at flow cells in the positive and negative electrolyte loop. When comparing the state of charge obtained from the different potential measurements the results suggested that monitoring the half-cell potentials of the battery enabled timely detection of crossover processes and furthermore the determination of the direction of crossover between the half-cells.
There are several advantages to using fluorescence as self-reporting agent on the health of redoxmer batteries, the most important one being that the material is highly sensitive. Thus, scientists can see the molecule the minute it crosses over. Other detection methods such as cyclic voltammetry or absorbance spectroscopy, which measure current or absorbance of light, may interfere with a running battery or have sensitivity limits. Also, the fluorescence offers a unique visual medium that makes the interactions with the other components of the battery fluid visible, such as the supporting electrolyte.
“We envision that we could apply this to most battery cycling parameters, such as capacity decay,” the scientists believe. Next, in-depth research will have to prove that this sentiment is true, and the technology is indeed applicable across other state-of-health metrics.