Aenert. Research Laboratory news
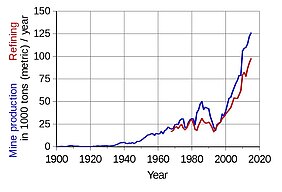
In search of more reliable devices for energy storage which are able to power homes, cell phones or e-vehicles, scientists are exploring possibilities of designing batteries without are and hard-to-provide elements. One of the major drawbacks of modern battery technology is that they rely heavily on a scarce metal: cobalt. Cobalt is mainly won in African mines, where environmental safety as well as labour rights are poorly regulated, which can lead to uncontrolled exploitation of both.
Now (2023), researchers at the Argonne National Laboratory have taken part in a collaborative study whose aim was to identify new potential materials for the positive cathode. During charging, lithium ions are inserted into a cathode and released during discharging, which produces electricity. As cobalt provides thermal stability, which means it functions even as it is heated to higher temperatures, as well as structural stability, the scientists were looking for a material having the same qualities.
They designed and analysed for a lithium-ion cathode which uses nickel instead of cobalt. The cathode composition is complex and contains several other metals including molybdenum, niobium and titanium.
In a next step, the researchers investigated the structural and thermal stability of the new cathode. Usually, nickel-rich cathodes exhibit poor heat tolerance, which can lead to oxidization of battery materials and thermal runaway, which could in some cases lead to explosions. Moreover, regardless of the fact that high-nickel cathodes can accommodate larger capacities, large changes in volume from repeated expansion and contraction can result in poor stability and safety concerns. In order to the its capacity, the scientists cycled the battery more than a thousand times. They found that during cycling the cathode material underwent less than 0.5% of volume expansion.
For further characterization of the heat tolerance of the new cathode material the team used beamline 11-ID-C to examine the behaviour of the material at high temperatures. As opposed to previous high-nickel cathodes, which underwent severe nanocracking at high temperatures, the HE-LMNO experienced a phase change that allows it to continue to perform and retain capacity.
Advancing battery technology has long been one of the focus areas of science. In 2020, scientists composed a AlF3-coating of the Li-rich Co-free layered Li1.2Ni0.2Mn0.6O2 (LLNMO) oxide.
Image: SEM-Image of LLMNO and AIF3-coated LLMNO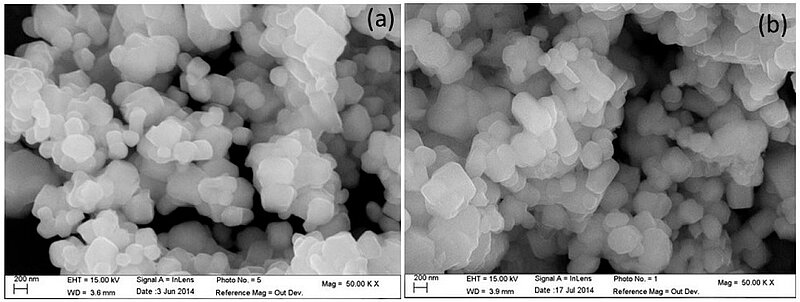
Source: Ashraf Abdel-Ghany, A.M.A. Hashem, Alain Mauger, Christian Julien/ Lithium-Rich Cobalt-Free Manganese-Based Layered Cathode Materials for Li-Ion Batteries: Suppressing the Voltage Fading/ Energies 13(13):3487, July 2020/ DOI:10.3390/en13133487/ Open Source This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
They synthesized the samples via the hydrothermal route which enabled very good crystallization in the layered structure, probed by XRD, energy-dispersive X-ray (EDX) spectroscopy, and Raman spectroscopy. Therefore, the hydrothermally synthesized samples were well crystallized in the layered structure before and after the coating having particle sizes of about 180 nm, with high porosity (pore size 5 nm) determined by Brunauer–Emmett–Teller (BET) specific surface area method.
The cathode was subsequently improved in discharge capacity with the help of a ~5-nm thick coating layer. AlF3-coated Li1.2Ni0.2Mn0.6O2 exhibited a capacity of 248 mAh g−1 stable over the 100 cycles, and a voltage fading rate of 1.40 mV per cycle. Post-mortem analysis confirmed that the AlF3 coating was an efficient surface modification to improve the stability of the layered phase of the Li-rich material.
Also in 2020, scientists showed that the dissolution of iron is mainly responsible for the low Coulombic efficiency of the iron-substituted cobalt-free lithium-rich material. Moreover, they found a method to reduce the dissolution of transition metal ions by using concentrated electrolytes. The cathode electrolyte interphase (CEI) layer formed in the concentrated electrolyte was a uniform and robust LiF-rich CEI. It was found to not only effectively inhibit the dissolution of TMs but also stabilisde the cathode structure. The Coulombic efficiency, cycling stability, rate performance, and safety of the Fe-substituted cobalt-free lithium-rich cathode material in the concentrated electrolyte could be improved significantly. In this experiment, the scientists inhibited metal dissolution with the help of highly concentrated electrolytes that induce ultra-thin cathode electrolyte interphase formation on cathode surfaces during cycling.
Image: Structural characterization and EDS spectra of the LNFMO a Rietveld refinement XRD pattern of LNFMO; b SEM image of the sample; c–e HRTEM images and corresponding FFT of the sample; f–k elemental distribution in LNFMO obtained from STEM-EDS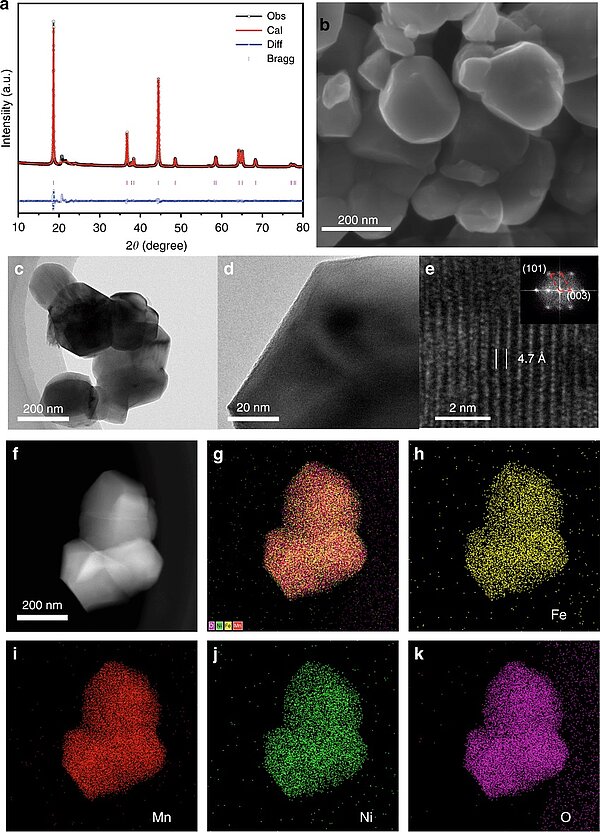
Source: Wei Liu, Jinxing Li, Li Wenting, Hanying Xu/ Inhibition of transition metals dissolution in cobalt-free cathode with ultrathin robust interphase in concentrated electrolyte/ Nature Communications 11(1), July 2020/ DOI:10.1038/s41467-020-17396-x/ Open Source This is an Open Access article is distributed under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0)
The new cathodes has two advantages over commonly-used ones: They are cobalt-free and stable. They do not undergo a structural failure, including cracking, because they are repeatedly charged and discharged.
The current research will advance design rules for a host of new battery cathodes and help reduce next-generation lithium-ion batteries’ reliance on cobalt. To get the best result, the researchers collaborated with researchers from national labs, industry and academia. This enabled them to construct baseline electrodes and validate promising chemistries using pilot scale equipment, which is critical for assessing advanced materials.
By the Editorial Board