Lithium is a mineral which occurs naturally and constitutes about 0.0007 percent of the Earth's crust. Lithium is contained in minerals and salts of natural brines which can be found underground or in seawater. Lithium can also form a byproduct of hydraulic fracturing. There it is mostly contained in fracturing fluids. Such brines and produced water also show high concentrations of rare earth elements and metals. Since the 1990s, lithium has been an important component of laptop batteries, phones as well as electric cars. Chile and Australia have the largest deposits of lithium in the world. As the demand of secure and efficient power storage is constantly rising, energy companies are increasingly turning to lithium production to avert possible future problems related to power shortages.
Now (2022), scientists at the National Energy Technology Laboratory have created a new CO2 injection technique to produce lithium carbonate in situ in a brine. The method consisted of heating a natural brine in a vessel and adding CO2 into the vessel which then combined with the natural brine. After keeping the mixture for a time when the CO2 had been added, a solid was precipitated from it.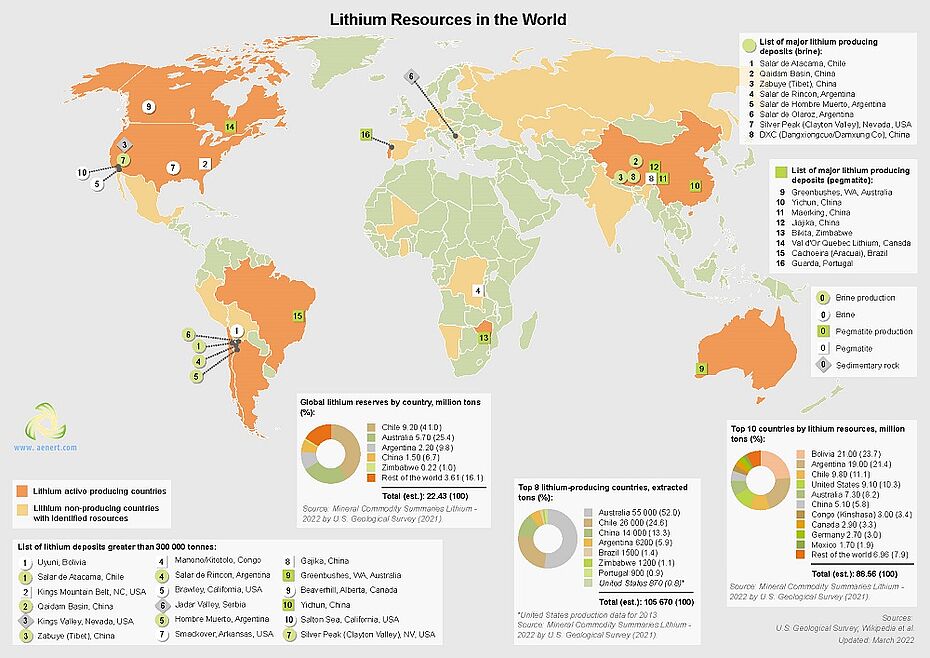
Then, the scientists separated the precipitated solid from the mixture, thus receiving a second brine which basically comprised the selected material. The second brine was then heated again and CO2 was also added to the vessel which mixed with the second brine and formed a second mixture. The second mixture was also held for a time after adding CO2 and a selected material was precipitated from the second mixture.
The CO2 used in the experimental setup was used directly from the air. However, it could potentially also be sourced from industrial waste streams. The production facility is portable so it could facilitate full operation at the source of brine, which would reduce the capital investments currently required in lithium production processes.
Lithium recovery from brine has attracted a lot of scientific attention lately. In 2021, scientists extracted lithium from geothermal brine suing a practical and environmentally friendly method. Experiments on the extraction of lithium resources from synthetic geothermal brine with a specific lithium composition using the electrodialysis (ED) method was then carried out. The ED device the scientists employed was fed with electricity and run using temperature variations (30°C and 40°C) and variations in electric voltage (2 V and 4 V). The highest flux was achieved at an operating temperature of 40°C and a power supply voltage of 4 V.
Image 1: Experimental apparatus for ED cell (1)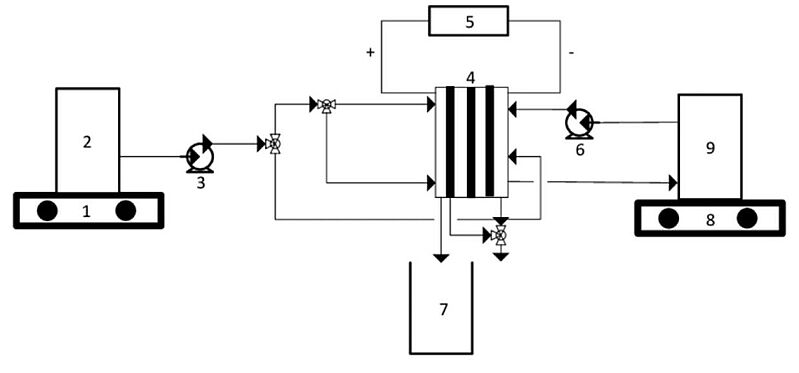
Source: Vincent Sutresno Hadi Sujoto, Widi Astuti, Fika Rofiek Mufakhir, Sutijan Sutijan/ Lithium recovery from synthetic geothermal brine using electrodialysis method/ IOP Conference Series Earth and Environmental Science 882(1):012003, November 2021/ DOI:10.1088/1755-1315/882/1/012003/ Open Source This article is licensed under a Creative Commons Attribution 3.0 Unported
In 2021, scientists analysed the thermo-economic comparison between organic Rankine cycle (ORC), which uses a fuel to produce heat within a boiler, thus turning water into steam which then expands through a turbine producing energy, and the binary-flashing cycle (BFC), which is a method for generating electrical power from geothermal resources and employs two separate fluid cycles, for geothermal energy. R245fa, a refrigerant, was selected as the working fluid. Several evaluation indicators were selected including thermal efficiency, exergy efficiency, net power output per ton geothermal water, heat exchanger area, and heat recovery efficiency, and the system modelling. The simulation results showed that the BFC system had 32% more net power output than the ORC system under the working conditions investigated. The heat recovery efficiency of the BFC was almost two times as much as that of the ORC, which indicated that the BFC could facilitate the full utilisation of low-grade energy. Also, a analysis of the economic feasibility of BFC system applied in the FengShun geothermal power plant was carried out. The payback period of the BFC estimated at 6.0 years under the generation pressure of 600 kPa which pointed to economic benefits of the BFC system, especially in a nonflowing geothermal well.
Image 2: Flow diagram of the ORC. Image 3: Flow diagram of the BFC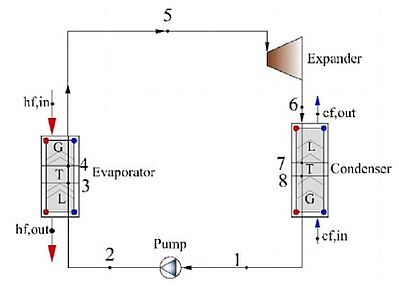
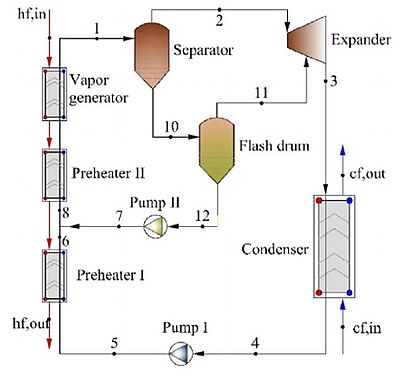
Source: Yuan Zhao, Bowen Du, Shunyi Chen, Jun Zhao/ Thermo-Economic Comparison Between Organic Rankine Cycle and Binary-Flashing Cycle for Geothermal Energy/ Frontiers in Earth Science 9:759872, October 2021/ DOI:10.3389/feart.2021.759872/ Open Source This article is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0)
There are several advantages to using the new method for producing lithium: the technique of direct lithium extraction does not need any adsorbents, absorbents, or membranes. There is no need to use freshwater, as well solid chemicals, acids, and a mineralization facility to produce the lithium minerals. CO2 can be added to a brine in such a way that mineralisation of a brine can quickly take place even under ambient conditions. This, in turn, enables fairly quick recovery of critical materials from brines while having a relatively small footprint and employing an environmentally benign fashion. Apart from natural brines and produced water, the process could extract lithium, rare earth elements and other critical materials from sea water. It could also be used as a tool for carbon sequestration and to clean contaminated water.
One World Lithium Inc. are currently evaluating the NETL patent (United States Patent: 10315926 (uspto.gov) and United States Patent Application: 0210047196 (uspto.gov)) for use on naturally occurring brines with the exception of sea water and geothermal brines, through a non-exclusive research and evaluation license.
