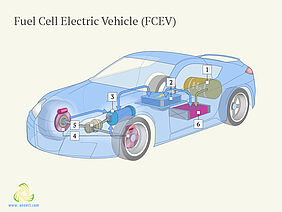
Cars running on hydrogen are one of the greatest hopes for achieving a cleaner environment. They are similar to electric vehicles because they also use an electric motor instead of an internal combustion engine. However, unlike electric vehicles they are able to produce their own electricity: in a fuel cell, hydrogen (H2) gas from the fuel tank combines with oxygen (O2) from the air to generate electricity while only water and heat are produced as waste products of the process. Thus, no harmful substances are generated, which would help reduce carbon dioxide and general air pollution levels. But although hydrogen is one of the most abundant elements on the planet, it is currently expensive to produce from non-fossil sources. One of the processes employed to receive so-called green hydrogen is water splitting, which is a clean and fairly sustainable process. Water splitting uses catalysts, which can increase the rate of a chemical reaction, but also undergo changes and decrease the efficiency of the reaction.
Now (2022), a team of scientists headed by Oregon State University (OSU) have demonstrated a way to produce hydrogen with much greater efficiency and at a lower cost. In their research, the scientists were particularly interested in the restructuring of catalysts, structural changes which they often experience during reaction. If these changes cannot be reversed, they are believed to reduce the ability of a catalyst to influence chemical reactions. The researchers studied the restructuring of catalysts in reaction and then modified their surface structure and composition at the atomic scale to achieve a highly efficient catalytic process for producing hydrogen. The new catalysts based on amorphous iridium hydroxide were 150 times more efficient than the original structures they were made from, and almost three times better than the common commercial iridium oxide catalyst.
The team of scientists confirmed their findings using several X-ray techniques at the Advanced Photon Source (APS). With the help of beamline 9-BM and 4-ID-C, the research team was able to study the electrochemical process as it happened, getting information about changes the catalyst experienced in real time, as APS enabled them to gain insights into what was happening on the surface of the materials, if they were eroding or transforming during reaction.
Image: Theoretical prediction of model perovskites’ surface stability. (A) A schematic shows that the dissolution of A-site Sr (the blue ball), from the subsurface layer of SSI to electrolyte, can be kinetically blocked by the cage composed of B-site (Ir/Sc) octahedra. The Sr atom away from the surface is considered as bulk Sr (the green ball). (B and C) Surface of SSI without (B) and with (C) a B-site (Sc) vacancy. (D and E) Energy diagrams that illustrate the dissolution of A-site (Sr) from the subsurface of SSI without (D) and with (E) a B-site (Sc) vacancy. In (B) to (E), for a better illustration, only the selected subsurface Sr atom, which migrates to the outer surface, is marked with blue color. All the rest of subsurface Sr atoms are marked with green color.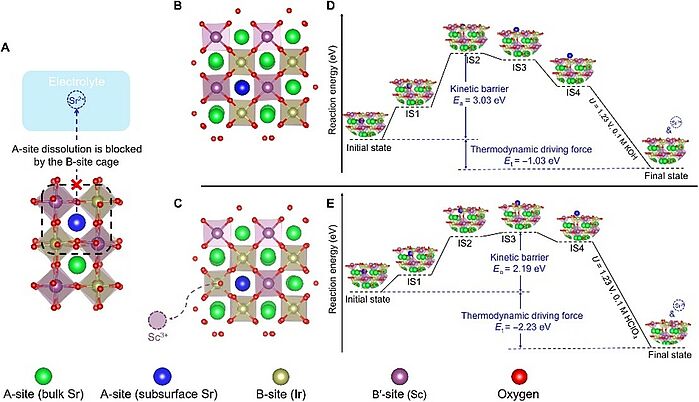
Source: Yubo Chen, Yuanmiao Sun, Maoyu Wang, Jingxian Wang, Haiyan Li, Shibo Xi, Chao Wei, Pinxian Xi, E. Sterbinsky, John W. Freeland, Adrian C. Fisher, Joel W. AgerIII, Zhenxing Feng and Zhichuan J. Xu/ Lattice site–dependent metal leaching in perovskites toward a honeycomb-like water oxidation catalyst/ Science Advances Vol 7, Issue 50, 10 Dec 2021 / DOI: 10.1126/sciadv.abk1788/ Open Access This article is licensed under a Attribution 4.0 International (CC BY 4.0)
Efficient hydrogen production has been afforded great scientific attention for many years. In 2018, scientists demonstrated high performance in a membrane electrode assembly (MEA) based on a non-precious metal catalyst, achieving a peak power of 570 mW/cm2 under air. The good performance was achieved by using a precommercial catalyst whose pores were less than 3 nm in diameter. This enabled them to disprove previous beliefs regarding the need for larger catalyst pores to achieve high current densities. The experiment was conducted at industrially relevant scales (50 cm2 MEA) using a precommercial non-precious metal catalyst. In situ electrochemical analysis of the cathode catalyst layers was also used to help gain insight into the degradation mechanism during galvanostatic testing.
Image: Theoretical prediction of model perovskites’ surface stability. (A) A schematic shows that the dissolution of A-site Sr (the blue ball), from the subsurface layer of SSI to electrolyte, can be kinetically blocked by the cage composed of B-site (Ir/Sc) octahedra. The Sr atom away from the surface is considered as bulk Sr (the green ball). (B and C) Surface of SSI without (B) and with (C) a B-site (Sc) vacancy. (D and E) Energy diagrams that illustrate the dissolution of A-site (Sr) from the subsurface of SSI without (D) and with (E) a B-site (Sc) vacancy. In (B) to (E), for a better illustration, only the selected subsurface Sr atom, which migrates to the outer surface, is marked with blue color. All the rest of subsurface Sr atoms are marked with green color.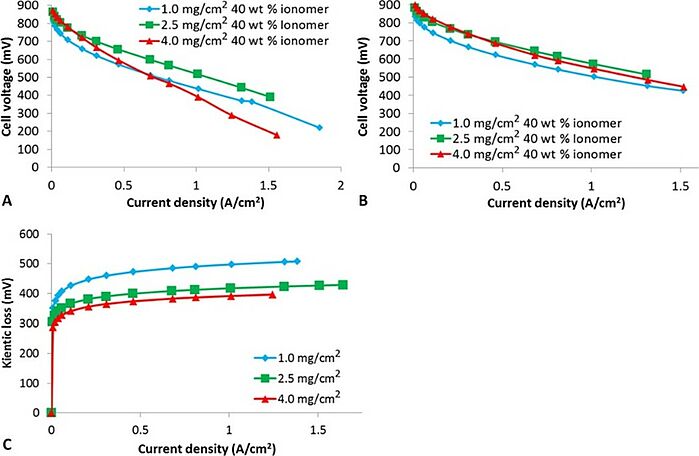
Source: Dustin Banham, Takeaki Kishimoto, Yingjie Zhou, Tetsutaro Sato, Kyoung BaiJun-ichi Ozaki, Yasuo Imashiro and Siyu Ye/ Critical advancements in achieving high power and stable nonprecious metal catalyst–based MEAs for real-world proton exchange membrane fuel cell applications/ Science Advances Vol 4, Issue 3, 23 Mar 2018/ DOI: 10.1126/sciadv.aar7180/ Open Access This article is licensed under a Attribution 4.0 International (CC BY-NC 4.0)
In February 2022, scientists analysed a family of nonprecious transition metal nitrides (TMNs) as oxygen reduction reaction electrocatalysts in an alkaline medium. The air-exposed nitrides spontaneously formed a several-nanometer-thick oxide shell on the conductive nitride core and served as a highly active catalyst architecture. The most active catalyst, carbon-supported cobalt nitride (Co3N/C), exhibited a half-wave potential of 0.862 V and achieved a record-high peak power density among reported nitride cathode catalysts of 700 mW cm−2 in alkaline membrane electrode assemblies. Using operando x-ray absorption spectroscopy studies they discovered that Co3N/C remained stable below 1.0 V but experiences irreversible oxidation at higher potentials. This work provided a comprehensive analysis of nonprecious TMNs as ORR electrocatalysts and was intended to help inform future design of TMNs for alkaline fuel cells and other energy applications.
Image: Synthesis and structural characterization of carbon-supported 3d metal nitrides. (A) Schematic synthesis procedure of metal nitrides (MxN) supported on high–surface area carbon via nitridation with ammonia. HMT, hexamethylenetetramine. (B to D) XRD patterns of as-synthesized MxN/C compared with patterns from Pearson’s Crystal Data (PCD) database and their atomic arrangement models with different structures. MnN/C, TiN/C, VN/C, CrN/C, Fe3N/C, Co3N/C, and Ni3N/C were prepared at 800°, 800°, 600°, 700°, 400°, 360°, and 300°C, respectively. a.u., arbitrary units.
Source: Rui Zeng, Yao Yang, Xinran Feng, Huiqi Li, Lauryn M. Gibbs, Francis J. DiSalvo and Héctor D. Abruña/ Nonprecious transition metal nitrides as efficient oxygen reduction electrocatalysts for alkaline fuel cells/ Science Advances Vol 8, Issue 5, 2 Feb 2022/ DOI: 10.1126/sciadv.abj1584/ Open Access This article is licensed under a Attribution 4.0 International (CC BY 4.0)
The new research insights might have great bearing on future hydrogen production: the scientists found that at least two groups of materials that undergo irreversible changes turned out to be significantly better catalysts for hydrogen production. This could enable hydrogen production at $2 per kilogram and eventually $1 per kilogram. X-ray absorption spectroscopy allowed them to look at the atomic structure and see how the catalyst was changing which gave them chemical and structural information to understand the catalytic process.
Catalysts are critical to promoting the water-splitting reaction because they can lower the overpotential of the reaction and thus also decrease the total cost for hydrogen production. The study was certainly an important step toward creating tailored catalysts through precise manipulation of surface atoms to gain catalysts with the desired structure and composition.