Solid-state batteries are considered an important power source for a wide variety of applications. They use solid electrodes and solid electrolytes instead of the liquid or polymer gel electrolytes usually found in commercial batteries. Materials proposed as solid electrolytes for this type of battery comprise ceramics (e.g., oxides, sulphides) and solid polymers. Solid-state batteries have the potential to provide a solution to many problems we now see in common liquid Li-ion batteries. They are also a promising candidate for large-scale industrial applications as well as energy storage devices. However, before this goal can be achieved, several technical problems need to be tackled, concerning, for example, energy and power density, durability, material costs, sensitivity and stability.
Now (2022), scientists at Sandia National Laboratory have taken a closer look at these problems, particularly the one concerning the assumed reduction in battery safety when some liquid electrolyte is added for the sake of improving performance. Instead, the scientists discovered that safety in solid-state batteries was actually improved with a little liquid electrolyte. They also found that in case of a short-circuit, the all-solid-state battery could reduce the amount of critical heat produced in the battery.
In order to prove their assumptions, the scientists calculated how much heat could be released in a lithium-ion battery, an all solid-state battery and solid-state batteries with varying amounts of liquid electrolyte. All batteries tested had equivalent amounts of stored energy. Then, they looked at three different bad things that could happen to the batteries, and the heat that would be released during by each type of failure, including: firstly, if the batteries caught fire, which, however, is not very likely as the solid-state battery containing only a little liquid electrolyte produce about one-fifth of the heat of a comparable lithium-ion battery; secondly, if repeated charging and discharging caused the lithium metal to form dendrites which can puncture a hole through the separator which causes a short-circuit; thirdly, if the solid electrolyte broke. This can happen if the battery is crushed or punctured or due to built-up pressure during operation, which would allow oxygen from one side of the battery to react with the lithium metal on the other side. The outcome of their research was that in general adding a bit of liquid could greatly increase performance while only having a small impact on reducing safety.
Scientists have long sought to improve solid-state batteries for large-scale commercial use. In 2019, a contact mechanics model for tracking Li surface and sub-surface stresses was designed. At the larger, sub-mm scales the contact between the Li metal and the separator was heterogenous and the load was carried at just the tallest asperities, where stresses reached tens of MPa, while most of the Li surface experienced no force at all. Still, dendrite growth was suppressed over the entire Li surface. The explanation for this phenomenon was that Li avoided plating at the tips of growing Li dendrites if there was enough local stress and that therefore local contact stresses there were high enough to shut separator pores so that Li+ ions could plate in another place.
Image: 3D structure of a lithium (Li) battery material system.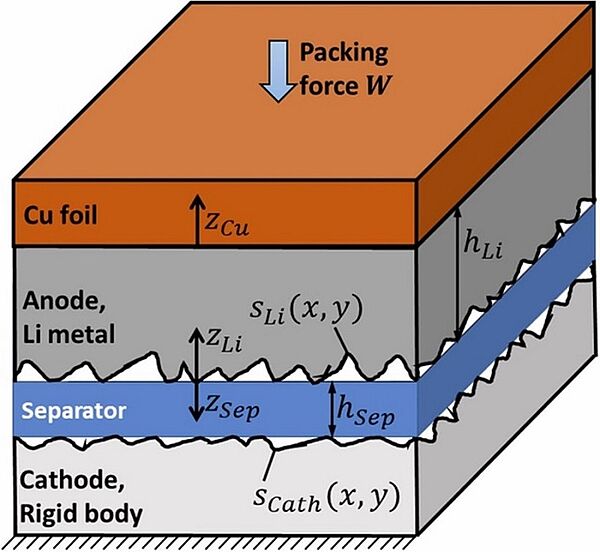
Source: Rachel Carter, Emily J. Klein, Todd A. Kingston and Corey T. Love/ Rethinking How External Pressure Can Suppress Dendrites in Lithium Metal Batteries/ Journal of The Electrochemical Society, Volume 166, Number 15, 4 November 2019/ doi.org/10.1149/2.0701914jes/ Open Access This article is licensed under a Attribution 4.0 International (CC BY 4.0)
In 2020, scientists proved that Li-metal anodes >20 μm could be electroplated onto a current collector in situ without degradation of the Li7La3Zr2O12 (LLZO) electrolyte. A full cell consisting of in situ formed Li, LLZO, and NCA (nickel cobalt aluminium oxides) was built, which showed stable cycling over 50 cycles and high Coulombic efficiencies. The findings showed that lithium-free configurations using LLZO were possible which could lead to the design and manufacturing of high energy density solid-state batteries.
Image: Cross-sectional SEM at the interface of the LLZO and the current collector. SEM (a) as assembled, (b) after plating 5 mAh cm−2 of Li, and (c) after plating and then stripping of 5 mAh cm−2 of Li. Elemental maps for Cu and Zr at the interface (d) as assembled, (e) after plating, and (f) after plating and stripping. Metallic Li is observed under secondary electrons in between the Cu and LLZO layer in b but cannot be detected since the characteristic X-ray energy falls outside of the detection range of EDS.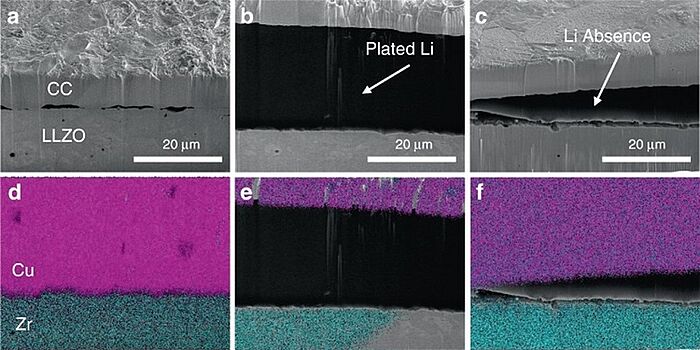
Source: Michael J. Wang, Eric Carmona, Arushi Gupta, Paul Albertus & Jeff Sakamoto/ Enabling “lithium-free” manufacturing of pure lithium metal solid-state batteries through in situ plating/ Nature Communications volume 11, Article number: 5201 (2020), 10 December 2020/ doi.org/10.1038/s41467-020-19004-4/ Open Access This article is licensed under a Attribution 4.0 International (CC BY 4.0)
There are several advantages to using solid-state batteries: solid-state batteries have the potential to be safer and also have higher energy density which means that electric vehicles, for example, could go farther or that fewer batteries for grid-scale energy storage would be needed. The addition of a little liquid electrolyte to a solid-state battery may help to bring about widespread commercialisation without having a negative effect on battery safety. Solid-state batteries are generally believed to be safer because they are firm and unlikely to break. However, if the battery does break, the temperature rise is the same as in commercial lithium-ion batteries.
The next steps of the project include conducting similar calculations with other solid electrolyte materials as well as experiments to validate the new and original calculations. The scientists are convinced that adding a bit of liquid may greatly increase the performance of batteries but only have a small impact on safety: “Having the clarity and the confidence that knowing a small amount of liquid electrolyte will not create huge safety issues may help the development of commercial solid-state batteries.”