Organic photochemistry is a scientific area encompassing processes which play an important role in enabling life on our planet. These processes have recently been afforded renewed scientific interest as it enables scientists to cheaply and effectively create new molecules. Gaining greater insights into already known processes can sometimes lead to incredible results and help design better materials than such that can be created by altogether new processes. Such is the case concerning a microwave technique which is almost 100 years old, but still able to astonish scientists by revealing new facets.
Recently (2022), scientists at the National Renewable Energy Laboratory (NREL) and Princeton University have discovered an astonishing new feature in a well-known light-driven chemistry. Their research analysed the mechanisms of operation in an important class of "photoredox catalysts", which led to unexpected ways to influence their efficiency and selectivity.
Photoredox catalysis is a branch of photochemistry which has received increased attention in recent years. This reaction uses light instead of heat or highly reactive chemicals to drive chemical reactions. This makes it possible to perform reactions with high kinetic or thermodynamic barriers and achieve better control over the final product as well as potentially need less harmful ingredients.
However, in order to be able to employ the new reaction in a large-scale setup, a detailed understanding of how the reaction works was needed. The catalyst studied by the NREL team was an ionic compound composed of two halves. One half had a positive charge, and the other a negative, so that the overall charge amounted to zero. In this catalyst, the positive side was believed to do all the work in light-driven chemical reactions, while the negative side was thought to be a supposedly inert "counterion." However, the scientists were proved wrong, when they found that the counterion actually moved after the catalyst had been excited by light, and that it could block certain kinds of reactions.
By measuring exactly in what manner their microwave signal changed when passing through the solution, the team showed that the negatively charged counterion moved after the molecule had been excited with light. This finding was important as molecules need a path for electrons to move through in order to start a chemical reaction which the counterion can block. The team discovered that the blocking action of the counterion led to a factor of four change between two different kinds of reactions.
In recent years, scientists have rediscovered the great potential of photoredox catalysts. In 2021, scientists analysed excited-state ion pair reorganisation in a cationic iridium (III) photoredox catalyst in 1,4-dioxane. Microwave-frequency dielectric-loss measurements gave the scientists the opportunity to assign both ground and excited-state molecular dipoles and excited-state polarizability volumes. The measurements showed significant changes in ground-state dipole moment. Photoexcitation of each complex caused population of highly mixed ligand centered and metal-to-ligand charge transfer states. Relaxation to the lowest lying excited-state led to a negative change as well as a positive change in dipoles. These observations were consistent with a subnanosecond reorganisation with the counter-ion. Taken together, these observations suggested contaction pair formation in cationic iridium (III) photoredox catalyst in 1,4-dioxane. The ion pair reorganisation which was observed was proved to modify both the thermodynamic potential available for electron transfer and inhibit oxidative catalysis.
Image: COMSOL Multiphysics electric-field amplitude solution for our solution-phase microwave resonance cavity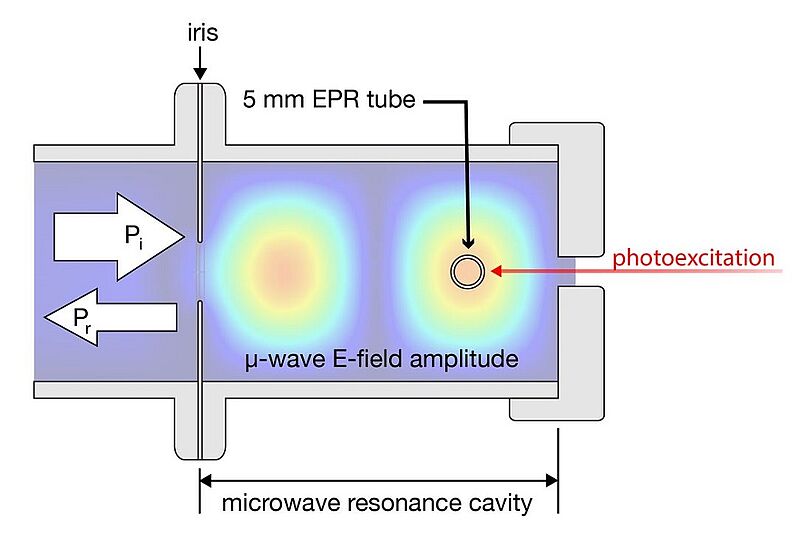
Source: Justin Earley, Anna Zieleniewska, Megan Lazorski, Zachary Mast, Robert Knowles, Gregory Scholes, Obadiah Reid, Garry Rumbles/ Dipole Moment and Charge Reorganization in Photoredox Catalysts/ US Department of Energy, Office of Science, Jun 25, 2021 Version 1/ 10.26434/chemrxiv-2021-cz105/ Open Access This article is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2022, scientists researched a new class of photoactive organic polymers which combined the flexibility of small-molecule dyes with the operational advantages and recyclability of solid-phase catalysts. The solubility of these polymers in select non-polar organic solvents enabled their facile processing into a wide range of heterogeneous modalities. The active sites, embedded within porous microstructures, showed elevated reactivity, further enhanced by the mobility of excited states and charged species within the polymers. The tunability of the physical and photochemical properties of these materials provided a medium for the metamorphosis of modern photoredox catalysts into active heterogeneous equivalents.
Image: Solution-processable microporous polymer platform for heterogenization of diverse photoredox catalysts: a Classification of photoredox catalysts according to solubility and geometric dimension of active surface. b Transforming a broad scope of small-molecule photocatalysts into diverse heterogeneous materials through solution-processable polymers
Source: Richard Y. Liu, Sheng Guo, Shao-Xiong Lennon Luo & Timothy M. Swager/ Solution-processable microporous polymer platform for heterogenization of diverse photoredox catalysts/ Nature Communications volume 13, Article number: 2775 (2022), 27 May 2022/ doi.org/10.1038/s41467-022-29811-6/ Open Access This article is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0)
There are several benefits to the outcome of the research: Although groundbreaking achievements were accomplished in this area of research, the task of transforming a specific C–H bond effectively and selectively under the right conditions (room temperature, without external oxidant, cost-effective, sustainable, and environmentally friendly) still is a very challenging issue to the science. In this context, visible light-induced photoredox catalysis, which is believed to be an abundant, inexpensive, renewable, and nonpolluting chemical transformation, has been the focus of increased scientific attention during the past years because of the extraordinary possibilities and exceptional reactivity pattern of the method.
Although the measurement tool used by the NREL team has existed and been used for over 100 years, in the past scientists had to conduct time-consuming control experiments to interpret their results. Now, the use of computers has simplified this task. The NREL team this time employed quantitative simulations of how the catalyst molecules rotated in solution and were thus able to interpret their results. Old techniques and new research methods formed the perfect team to open up the path for creating new innovative devices.