Ethane and particularly CO2 are gases which have long been recognised for their potential value as sustainable sources of energy. However, the processes to convert them into the desired products can be quite challenging to control. This is mainly due to the potentially unpredictable course of the reaction. Therefore, a suitable catalyst is of utmost importance for the correct progress of the reaction. However, so far scientists have still been experiencing problems with steering the selectivity of the process.
Now (2022), scientists at Brookhaven National Laboratory have analysed what features determine catalytic selectivity for transforming CO2 and ethane (C2H6) into synthesis gas which can be used for generating electricity or making liquid fuels or, alternatively, ethylene which is a constituent of paint and plastics. For this reason, the team conducted studies of several bimetallic catalysts consisting of different metals and paired with palladium. For each metal combination, they analysed the interaction of the metals and measured how the mix of reactants and products changed during the reaction. They also looked at the atomic structures and electronic properties of the catalysts using x-rays. Moreover, they ran computational modelling studies including “density functional theory” (DFT) to predict how the arrangement of atoms making up the catalyst changed as the reaction transpired, analysing the binding energies between different sets of atoms and what energies were needed to break and remake chemical bonds. The result of their research was the discovery of two key principles, or descriptors, that were responsible for the movement of the metal atoms, and also the influence they had on the shifts in reaction selectivity, as well as the “formation energy” of the bimetallic catalyst, i.e. how tightly bound together the two metals are, and the binding energy between the catalyst and oxygen released from the CO2 during the reaction. For example, when the two metals were bound together strongly (e.g., palladium paired with cobalt), the catalyst would not bind with the freed oxygen and would remain an alloy. This led to the breaking of carbon-carbon bonds and the selectively transforming of CO2 and ethane into carbon monoxide and hydrogen gas, the constituents of syngas. However, if the affinity for freed oxygen in a catalyst is strong enough to overcome the formation energy of the alloy (palladium paired with indium) the paired metal will move to the surface of the catalyst to form an oxide shell. This configuration was prone to breaking carbon-hydrogen bonds, driving the pathway that could produce ethylene. The other metals which were paired with palladium behaved between these two extremes.
Scientists have long been working to find methods to transform harmful substances into useful fuels. In 2020, a method to produce C3 oxygenates with the help of a tandem reactor was devised. For this purpose, they used a Fe3Ni1/CeO2 catalyst in a first reactor at high temperature for CO2-assisted dehydrogenation and reforming of ethane to produce ethylene, CO, and H2, as well as a RhCox/MCM-41 catalyst in a second reactor at low temperature, which enabled CO insertion for the production of C3 oxygenates (propanal and 1-propanol) via the heterogeneous hydroformylation reaction at ambient pressure. The method was aimed at upgrading light alkanes in shale gas by reacting with CO2 to produce aldehydes and alcohols.
Image: Catalytic performance of different catalysts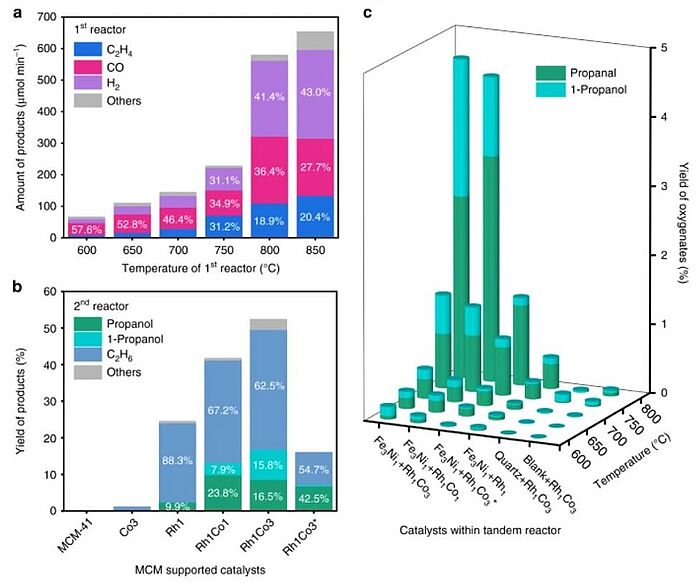
Source: Zhenhua Xie, Yuanguo Xu, Meng Xie, Xiaobo Chen, Ji Hoon Lee, Eli Stavitski, Shyam Kattel & Jingguang G. Chen/ Reactions of CO2 and ethane enable CO bond insertion for production of C3 oxygenates/ Nature Communications volume 11, Article number: 1887 (2020), 20 April 2020/ DOI:10.1038/s41467-020-15849-x/ Open Access This article is licensed under a Creative Commons Attribution 4.0 International License
In 2021, scientists designed a method for sunlight-driven thermo-photocatalytic reduction of carbon dioxide (CO2) by ethane (C2H6), which was contained in shale gas, to generate ethanol (EtOH). For this reason, a hybrid catalyst was designed which comprised strontium titanate (SrTiO3) co-doped with ruthenium oxide (RuO2) and nickel oxide (NiO). The photocatalytic activity regarding EtOH production was assessed in batch-mode and at gas-phase, under the influence of different conditions, such as dopant loading, temperature, optical radiation wavelength, consecutive uses, and electron scavenger addition. The research showed that the functionalization of the SrTiO3 with RuO2 and NiO was responsible for a visible light harvest and narrowed the band gap energy (ca. 14–20%) as well as that the selectivity towards EtOH depended on the presence of Ni and irradiation. Moreover, the scientists found that the reaction mechanism was founded on the photogenerated electron-hole pair charge separation. Finally, a maximum yield of 64 μmol EtOH gcat−1 was obtained after 45-min (85 μmol EtOH gcat−1 h−1) of simulated solar irradiation (1000 W m−2) at 200°C.
Image: Schematic representation of the SrTiO2:RuO2:NiO photocatalyst preparation method
Source: Larissa O. Paulista, Josep Albero, Ramiro J. E. Martins, Rui A. R. Boaventura, Vítor J. P. Vilar, Tânia F. C. V. Silva and Hermenegildo García/ Turning Carbon Dioxide and Ethane into Ethanol by Solar-Driven Heterogeneous Photocatalysis over RuO2- and NiO-co-Doped SrTiO3 / Catalysts 2021, 11(4), 461, 1 April 2021/ doi.org/10.3390/catal11040461/ Open access This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license
There are several benefits to the discoveries made in this study: identifying the descriptors influencing the formation of products, the scientists can now tailor the design of catalysts to the needs of different feedstock to make either syngas or ethylene. Moreover, the descriptors are generalised, which means they can be applied to many different catalysts. The experiments and theory showed that this approach worked for palladium-based catalysts, but it could possibly be used with other bimetallic catalysts as well.
If the assumptions of the scientists are correct, this would open the path for the production of many different fuel applications as well as reduce CO2 emissions in many areas where they are now released into the atmosphere almost unchecked.