As we become ever more dependent on lighter, thinner, and smaller electrical devices, the production and use of lithium-ion batteries has turned into one of the most important branches of industry, as they are needed for e.g. mobile phones and laptops, but also for larger applications such as electric vehicles. To ensure good and reliable performance, the makeup of the battery is essential. Battery performance and safety are closely related to the properties of the electrolytes used. Therefore, electrolytes often get enhanced with different additives depending on the qualities you want to achieve. The additives for lithium-ion batteries on the market can be roughly divided into three categories: improving cycle life, improving safety, and improving ion transmission characteristics based on their efficacy.
Now (2022), scientists at Brookhaven Lab have found an electrolyte additive which enables stable high-voltage cycling of cathodes, especially nickel-rich layered cathodes. Originally, the team of scientists was trying to find methods to use improve low-temperature performance of batteries with the help of an additive, lithium difluorophosphate (LiPO2F2). Out of curiosity, they also tried the additive for high voltage cycling at room temperature.
One of the main problems with high-voltage cycling is that nickel-rich layered cathode materials have high energy density when paired with lithium metal anodes but are susceptible to particle cracking during high-voltage charge-discharge cycles. Another problem is transition metal dissolution from the cathode and its subsequent deposition on the anode, which is known as crosstalk. During high-voltage charging, some of the transition metals in the cathode crystal lattice dissolve, travel through the electrolyte, and deposit on the anode side. As a result, both the cathode and anode degrade which leads to poor battery capacity retention.
In the current research, the scientists discovered that through introducing a small amount of additive to the electrolyte crosstalk can be prevented. When the additive decomposes, it turns into lithium phosphate (Li3PO4) and lithium fluoride (LiF) to form a protective cathode-electrolyte-interphase which is a solid thin layer that forms on the cathode during cycling. The electrolyte additive makes it possible to cycle a nickel-rich layered cathode at high voltages and increase the energy density while still maintaining 97 percent of its initial capacity after 200 cycles.
However, increased performance was not the only outcome of the research. Common nickel-rich cathodes are formed of polycrystals, which can be responsible for causing particle cracks and eventual decreased capacity. The study led the scientists to the assumption that using additive engineering can also effectively address the cracking issue in polycrystalline materials. In order to get a better understanding of the way the additive decomposes and protects the cathode surface, the researchers carried out a series of synchrotron experiments, including Quick X-ray Absorption and Scattering (QAS), Submicron Resolution X-ray Spectroscopy (SRX), In Situ and Operando Soft X-ray Spectroscopy (IOS), X-ray Powder Diffraction (XPD). They also collaborated with the European Synchrotron Radiation Facility in Grenoble, France and used x-rays to look at the morphology and the chemistry of different electrode particles, allowing scientists to anaylse defects and energy density. Also, imaging and computational studies helped the team identify how the additive works.
Scientists have long sought to improve battery performance through additives. In 2018, several ternary lithium-salt/ionic liquid/polyionic liquid electrolytes were analysed for synergies of bis(fluorosulfonyl)imide and bis(trifluoromethane)sulfonimide anions, primarily concerning their physico-chemical properties, with the intention of using them in high-temperature lithium batteries operating at 80°C. The electrolytes all showed low triglyceride and were thermally stable below 100°C. With bis(trifluoromethane)sulfonimide anions as the exclusive anion the electrolytes had higher thermal stabilities ≥125°C. The evolution of the interfacial resistance was shown to increase for all bis(fluorosulfonyl)imide and bis(trifluoromethane)sulfonimide anions during the first 40 h, but generally decreased with increasing Li-salt content. Also, the bis(fluorosulfonyl)imide and bis(trifluoromethane)sulfonimide anions with high Li-salt content were shown to have excellent performance. Long-term cycling at 0.5 C, however, exhibited less stable discharge capacities for 100 cycles, for the bis(fluorosulfonyl)imide and bis(trifluoromethane)sulfonimide anions with high Li-salt content. All in all, the presence of both anions in the lithium-salt/ionic liquid/polyionic liquid electrolytes had lower ionic conductivities and decreased thermal stabilities, but also showed higher oxidation stabilities and reduced interfacial resistances which was responsible for an improved rate capability, but inhibited long-term capacity retention.
Image: Chemical structures of poly (DDA), Pyr14, FSI and TFSI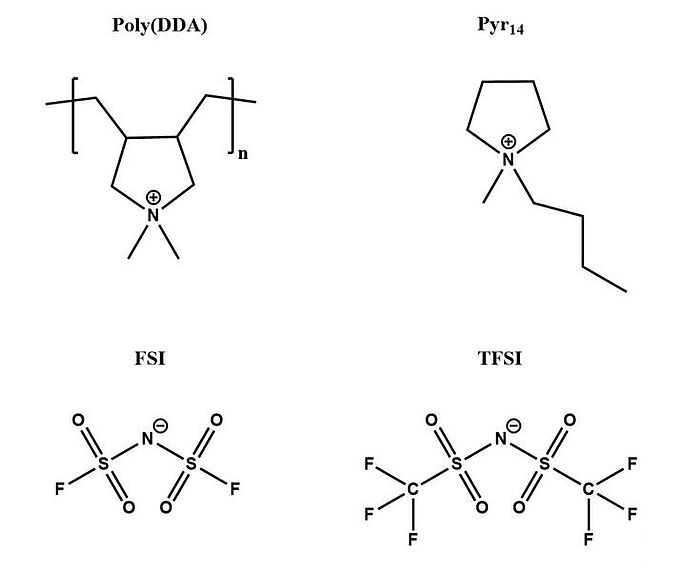
Source: Manfred Kerner, Patrik Johansson/ Pyrrolidinium FSI and TFSI-Based Polymerized Ionic Liquids as Electrolytes for High-Temperature Lithium-Ion Batteries/ Batteries 4(1):10, February 2018/ DOI:10.3390/batteries4010010/ Open Source This article is licensed under a Creative Commons Attribution 4.0 International
In 2021, scientists came up with an innovative synthetic additive that allowed for building a very stable electrode-electrolyte interface architecture from fluorinated and silylated electrolyte additives which could accommodate the lithiation-induced volume expansion of Si-embedded anodes and provide ion channels for easy Li-ion transport while protecting the Ni-rich lithium cathodes. The retrosynthetically designed solid electrolyte interphase-forming additives, 5-methyl-4-((trifluoromethoxy)methyl)-1,3-dioxol-2-one and 5-methyl-4-((trimethylsilyloxy)methyl)-1,3-dioxol-2-one, afforded spatial flexibility to the vinylene carbonate-derived solid electrolyte interphase through polymeric propagation with the vinyl group of vinylene carbonate. High-energy-density lithium-ion batteries with 81.5% capacity retention after 400 cycles at 1 C and fast charging capability (1.9% capacity fading after 100 cycles at 3 C) were achieved through the interface architecture from the synthesised vinylene carbonate-type additive.
Image: Synthesis of functional VC derivatives and transformation of additives to form SEI on the Si–C anode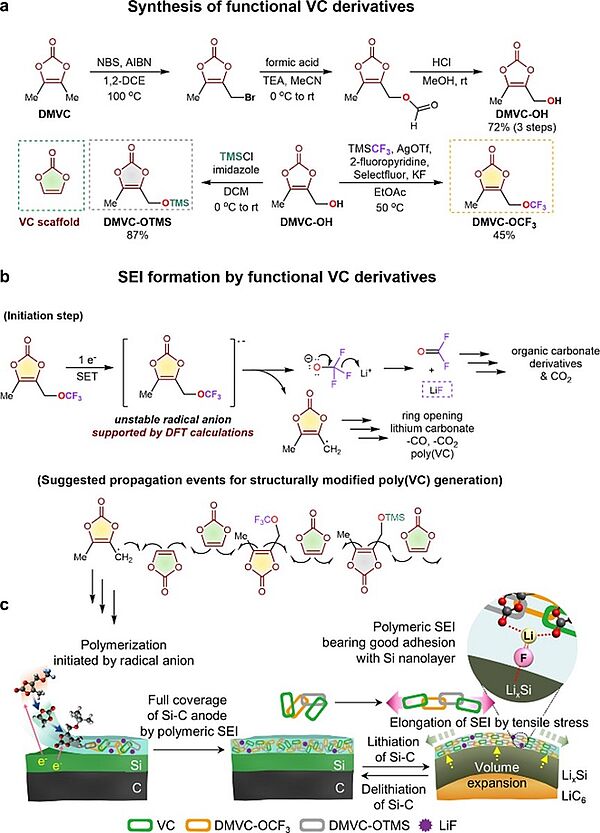
Source: Sewon Park, Seo Yeong Jeong, Tae Kyung Lee, Min Woo Park, Hyeong Yong Lim, Jaekyung Sung, Jaephil Cho, Sang Kyu Kwak, Sung You Hong & Nam-Soon Choi/ Replacing conventional battery electrolyte additives with dioxolone derivatives for high-energy-density lithium-ion batteries/ Nature Communications volume 12, Article number: 838 (2021), Published: 05 February 2021/ doi.org/10.1038/s41467-021-21106-6/ Open Source This article is licensed under a Creative Commons Attribution 4.0 International
There are several advantages to using additives in lithium-ion batteries: the scientists found that if the voltage was increased up to 4.8 volts (V), the additive afforded great protection over the cathode and the battery had very good cycling performance. By forming a stable interphase on the cathode, the protective layer can reduce the transition metal loss on the cathode surface. This decreased transition metal loss, in turn, enables reducing the deposition of transition metals on the anode. The scientists are convinced that suppression of transition metal dissolution is one of the main factors which can lead to significantly improved cycling performance. Also, with this new technique polycrystalline materials could be utilised, which are easier to make and therefore also cheaper.
With the new method only a very small amount of additive is used, which, however, leads to a great improvement of the electrochemical performance. These results are encouraging that there will be, in the end, a low-cost and easily-adoptable solution to the problem of sustainable high-voltage battery performance.