Cobalt (Co) is a chemical element and ferromagnetic metal of Group 9 (VIIIb) of the periodic table which is used especially for heat-resistant and magnetic alloys. Because the metal makes up only 0.001 percent of Earth’s crust and is high demand around the globe, especially for use in electronic devices which require batteries, it is one of the most significant supply chain risks threatening widespread adoption of electric cars, trucks and other electronic devices.
Cobalt is often received as a by-product in the course of mining operations to recover iron, nickel, copper, silver, manganese, zinc, or arsenic, which can all contain traces of cobalt. The ores are then further processed to concentrate and extract the metal. During the second decade of the 21st century, the Democratic Republic of the Congo (DRC), China, Canada, and Russia were the world leaders in mined cobalt production. The largest producer of refined cobalt was China, which imported vast additional amounts of cobalt mineral resources from the DRC. In view of the current COVID- and war-related supply chain issues, finding equally-efficient materials which can replace cobalt in batteries has become one of the most challenging issues of our time.
Now (2022), scientists at Brookhaven National Laboratory, the University of California, Irvine, and other national laboratories have devised a method to make lithium-ion battery cathodes which do not contain cobalt and use nickel instead. Thus, by simply mixing in a variety of other metallic elements they were able to overcome the thermal and chemical-mechanical instabilities of nickel cathodes.
Employing a technique called ‘high-entropy doping’, a cobalt-free layered cathode with high heat tolerance and stability over repeated charge and discharge cycles was successfully created. The doping method comprised HE-LMNO, a combination of the transition metals magnesium, titanium, manganese, molybdenum and niobium in the interior, with a subset of these minerals used on the surface and interface with other battery materials.
An array of synchrotron X-ray diffraction, transmission electron microscopy and 3D nanotomography instruments was used to show that the zero-cobalt cathode exhibited an unprecedented volumetric change of zero during repeated use. The structure had high stability and was capable of withstanding more than 1,000 cycles and high temperatures.
To prove the efficiency of the new cathode, the scientists relied on the advanced technology of the National Synchrotron Light Source II, located at the U.S. Department of Energy’s Brookhaven National Laboratory in New York. The combination of the different methods at the NSLS II beamlines enabled the discovery of a trapping effect of oxygen vacancies and defects inside the material, which effectively prevented the formation of cracks in the HE-LMNO secondary particle, making the structure extremely stable during cycling.
For many years, scientists have tried to improve lithium batteries and thus enhance their performance. In 2018, scientists used in situ transmission electron microscopy to demonstrate coupling of electrochemically triggered thermal and mechanical effects. They found that thermally disturbing delithiated LiNi0.6Mn0.2Co0.2O2 could trigger explosive nucleation and propagation of intragranular cracks in the lattice. This gave them the opportunity to directly visualise the cracking mechanism and dynamics. They were able to prove that thermal stress caused by electrochemically induced phase inhomogeneity and internal pressure resulting from oxygen release were mainly responsible for intragranular cracking that resembled a “popcorn” fracture mechanism. The study showed that, for battery performance, this intricate coupling of electrochemical, thermal, and mechanical effects could supersede the individual effects.
Image: Coupling of electrochemically triggered thermal and mechanical effects to aggravate failure in a layered cathode: Cycling performance and cracks of LiNi0.6Mn0.2Co0.2O2. a Capacity decay and b corresponding charge–discharge voltage profile evolution at cycling window 2.7–4.8 V. Cross-sectional high angle dark field (HAADF) images from (c) pristine sample d, e after 100 cycles at 2.7–4.5 V, f–h after 100 cycles at 2.7–4.8 V. Red arrow in (d) highlights intergranular cracks formed during cycling. Red arrows in (f, g) highlight some intragranular cracks within primary particles. Yellow arrows in (g, h) highlight incubation cracks. The scale bars are 500 nm in (c–f), 10 nm in (g) and 2 nm in (h)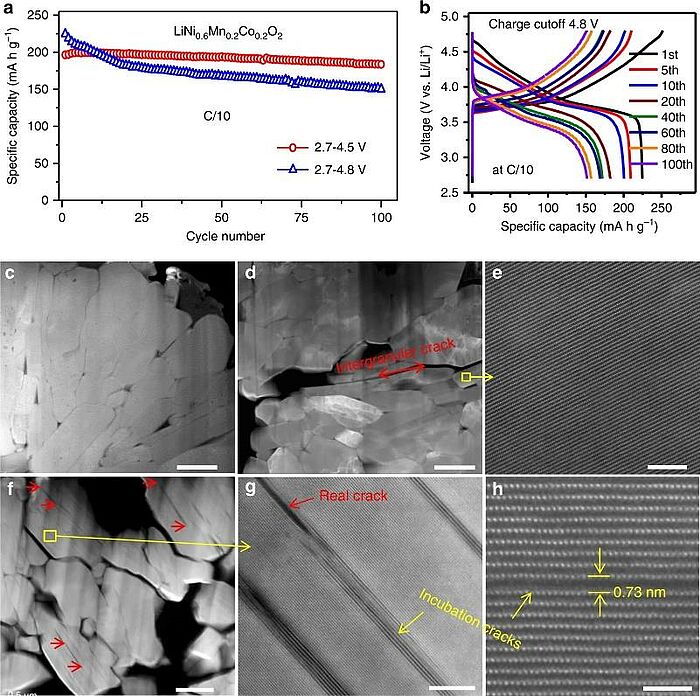
Source: Pengfei Yan, Tianwu Chen, Jianming Zheng, Tianwu Chen, Langli Luo/ Coupling of electrochemically triggered thermal and mechanical effects to aggravate failure in a layered cathode/ Nature Communications 9(1), June 2018/ DOI:10.1038/s41467-018-04862-w/ Open Source This article is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0)
In 2019, scientists used modern microscopy tools together with synchrotron X-ray techniques and first-principle calculations to look at a 4d-element-containing compound, Li2Ru0.5Mn0.5O3. They found that, after cycling, ruthenium segregated out as metallic nanoclusters on the reconstructed surface. Their calculations showed that the ruthenium metal segregation was caused by its thermodynamic insolubility in the oxygen-free surface. This insolubility could disrupt the reconstructed surface. The research highlighted how important it was to study the thermodynamic stability of the reconstructed film on the cathode materials and gave a theoretical guidance for choosing manganese-substituting elements in lithium-rich as well as stoichiometric layer-layer compounds for stabilising the cathode surface.
Image: Anomalous metal segregation in lithium-rich material provides design rules for stable cathode in lithium-ion battery: Electrochemical, X-ray pair distribution function and absorption analysis of Li2Ru0.5Mn0.5O3 (LRMO). a Charge/discharge curves of LRMO/lithium half-cell with cutoff voltages of 2 V and 4.7 V. b Charge/discharge capacity and coulombic efficiency of LRMO half-cell as a function of charge cycle. c Synchrotron X-ray powder diffraction measurement of pristine LRMO (inset: atomic model of LRMO). d X-ray pair distribution function of pristine LRMO, LRMO after 20 cycles, and Li2RuO3 as reference samples. e Hard X-ray absorption spectra of the pristine LRMO and LRMO after 15 cycles for the Ru K edge. f Soft X-ray absorption spectra of the pristine LRMO and LRMO after 15 cycles for the Mn L3 edge.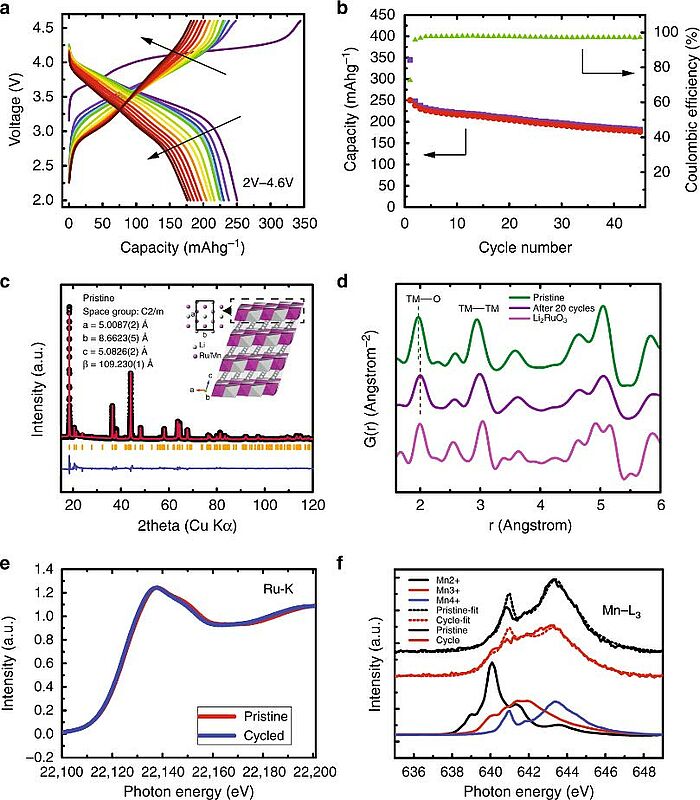
Source: Ruoqian Lin, Enyuan Hu, Mingjie Liu, Yi Wang, Hao Cheng, Jinpeng Wu, Jin-Cheng Zheng, Qin Wu, Seongmin Bak, Xiao Tong, Rui Zhang, Wanli Yang, Kristin A. Persson, Xiqian Yu, Xiao-Qing Yang & Huolin L. Xin/ Anomalous metal segregation in lithium-rich material provides design rules for stable cathode in lithium-ion battery/ Nature Communications volume 10, Article number: 1650 (2019)/ doi.org/10.1038/s41467-019-09248-0/ Open Source This article is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0)
There are several benefits which can be derived from cobalt-free cathodes: In-situ heating experiments showed that the thermal stability of the new cathode was significantly improved, reaching the level of the ultra-stable Lithium-Nickel-Mangan-Cobalt-Oxide. Because of the heightened thermal stability and the minor volumetric change, the cathode exhibited greatly improved capacity retention. By tackling and solving the long-standing safety and stability problems of high-Ni, zero-Co cathode materials, the research enables designing of a commercial cathode for safe, long-life lithium-ion batteries and presents a strategy for limiting strain and phase transformation in intercalation electrodes.
The scientists believe in the great potential of their discovery: “Using these advanced tools, we were able to observe the dramatically increased thermal stability and zero-volumetric-change characteristics of the cathode, and we’ve been able to demonstrate extraordinarily improved capacity retention and cycle life. This research could set the stage for the development of an energy-dense alternative to existing batteries.”
This research may prove a significant step toward speeding up the development of clean transportation and energy storage while also tackling environmental justice issues surrounding the extraction of minerals used in batteries.